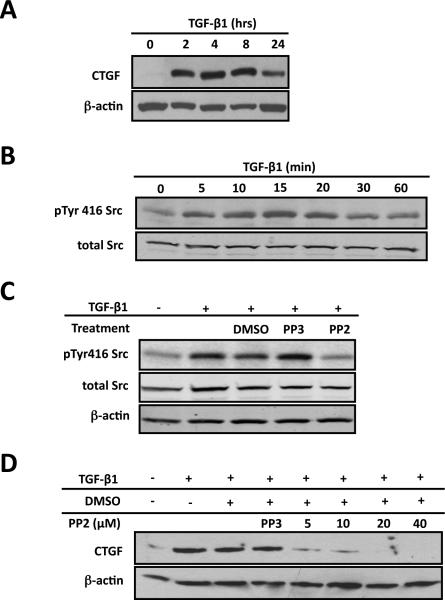
Figure 1. Src signaling is required for CTGF induction in ROS cells.
ROS osteoblast-like cells were cultured until they were 80% confluent and serum deprived for 24 hours before any treatment. After all treatments, whole-cell protein lysates were used for Western blot analysis. Each experiment was repeated a minimum of three times with similar results. (A) To determine if TGF-β1 induces CTGF expression cells were treated with TGF-β1 (5ng/ml) for indicated times. Whole cells lysates were extracted and used for Western blot. (B) To determine the time course for Src family kinase activation, serum-deprived cells were treated with TGF-β1 (5ng/ml) for 0, 5, 10, 15, 20, 30 or 60 minutes. Src kinase activation reaches maximal level 20 minutes following TGF-β1 treatment. (C) To determine if PP2 blocks Src activation in ROS cells, cells were serum-starved cells and pre-treated with 20mM PP2 (Src kinase inhibitor) or PP3 (inactive analog, negative control), or with DMSO (the diluent for PP2 and PP3) for 30 minutes and then treated with TGF-b1 (5ng/ml) for 20 minutes. Western blot analysis demonstrated that PP2 prevents TGF-β1 induced Src activation compared to controls. (D) To determine if Src signaling is required for CTGF induction cells were pre-treated with PP2 (at indicated concentrations), DMSO (the diluent for PP2) or PP3 (20mM; the inactive analog of PP2) for 30 minutes followed by TGF-β1 (5ng/ml) 4 hours. PP2 blocks the induction of CTGF expression by TGF-β1 (maximal effect at a dose of 20 μM) in primary and ROS osteoblasts.