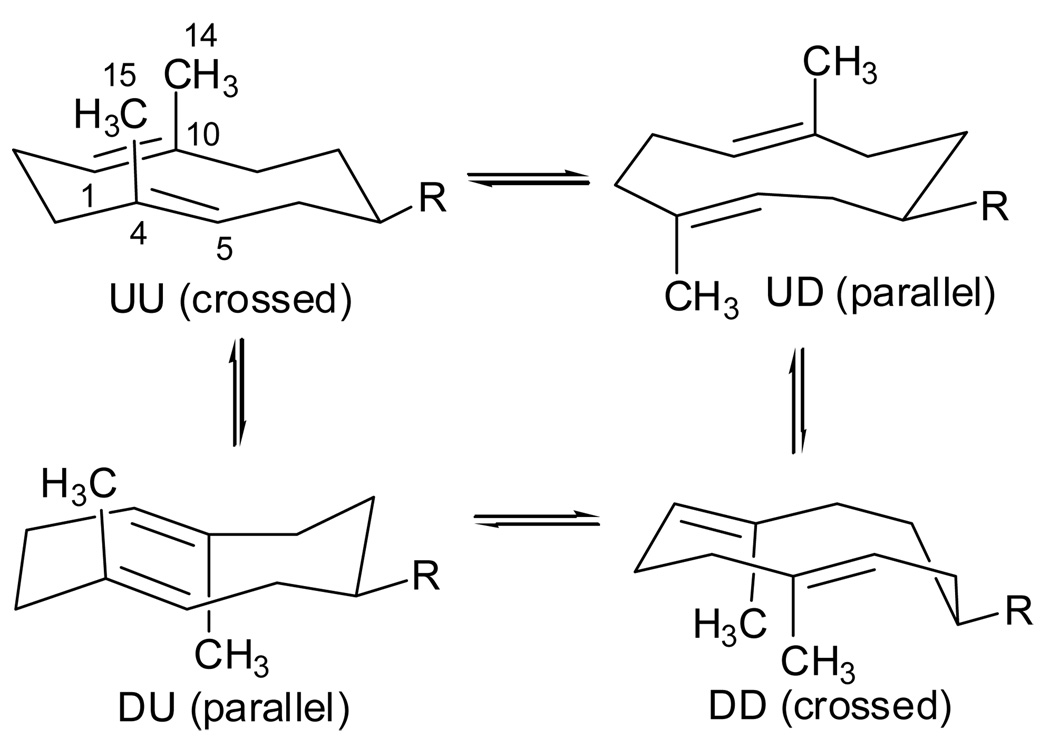
Figure 4.
Possible conformations of germacradiene sesquiterpenes such as germacrene A (1) and hedycaryol (2) fixed by the pseudo-equatorial position of the relatively large substituents (R = isopropenyl and hydroxypropyl). The conformers are denoted as UU, UD, DU and DD in reference to the U (up) and D (down) orientations of C10 and C4 methyl groups on the ten-membered ring.34, 35b