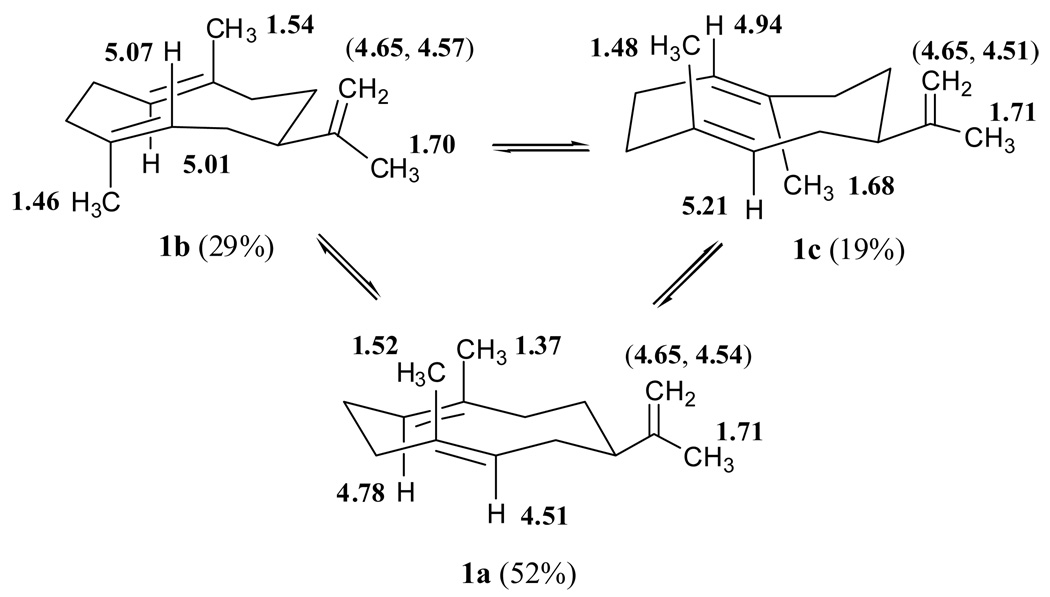
Figure 5.
The three conformers observed in the proton NMR spectra of germacrene A, 1a (52%), and 1b (29%) and 1c (19%), are assigned to one major crossed form (UU) and two minor parallel arrangements (UD and DU. The characteristic 1H NMR chemical shifts and assignments of hydrogens 1, 5, 12, 13, 14 and 15 of each conformer are shown by the placement of the bold numbers (see also Table 3).