Table 1.
Silylene transfer to α-keto esters with substitution at R1
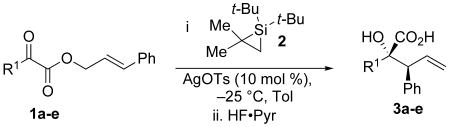 | ||||
|---|---|---|---|---|
| Entry | R1 | Product | % Yield | d.r. |
| 1 | Me | 3a | 70 | ≥97:3 |
| 2 | Et | 3b | 84 | ≥97:3 |
| 3 | i-Pr | 3c | 54 | ≥97:3 |
| 4 | t-Bu | 3d | 47 | ≥97:3 |
| 5 | Ph | 3e | 71 | ≥97:3 |
Conditions: α-keto ester (1.0 equiv), silacyclopropane 2 (1.5 equiv), AgOTs (0.10 equiv), toluene, −25 °C, 16 h. Then HF·Pyr (4.0 equiv), isolated yields.
