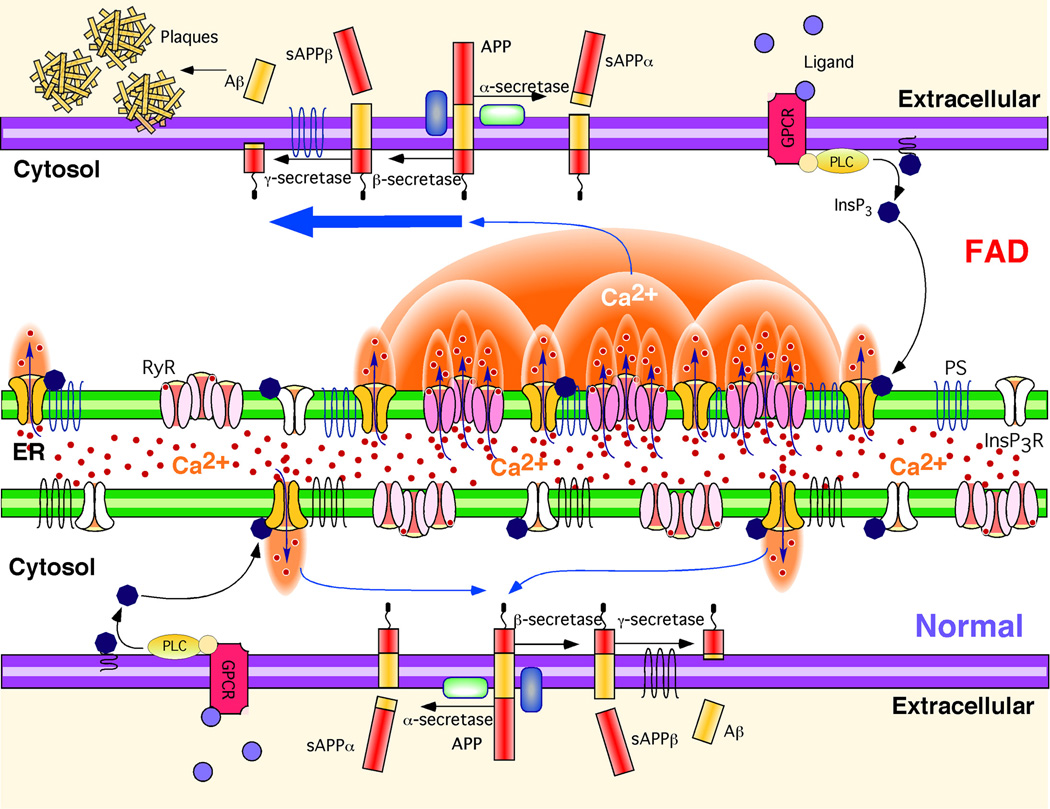
Fig. 7. Hypothetical molecular mechanism of enhanced Aβ production due to Ca2+ disruption in FAD PS cells.
APP is processed by either α-secretase or β-secretase, the latter leading to Aβ generation after subsequent cleavage by γ-secretase. Stimulation of G-protein coupled receptors (GPCR) or other cell surface receptors by extracellular ligands activates phospholipase C (PLC), which cleaves phosphatidylinositol bisphosphate (PIP2) to produce InsP3. InsP3 binds to and activates the InsP3R to release Ca2+ from ER stores, increasing cytoplasmic Ca2+ concentration. In normal cells, these Ca2+ signals are tightly regulated in time, space, and amplitude. In FAD cells, mutant PS exerts stimulatory effects on InsP3R gating by modal switching to the H mode associated with prolonged channel openings. H mode gating generates exaggerated Ca2+ signaling by promoting additional release channel recruitment by CICR. Increased cytosolic Ca2+ concentration promotes β-secretase activity (52) and Aβ production (51, 54), which, together with mutant PS-enhanced production of amyloidogenic Aβ, results in plaque formation.