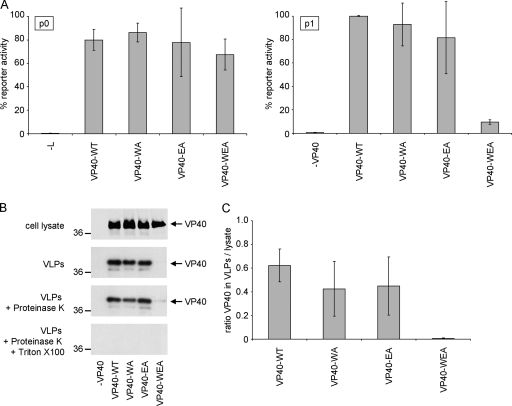
FIG. 3.
Influence of VP40 oligomerization on production of infectious VLPs. (A) Viral transcription/replication in an iVLP assay. 293 producer cells (p0) were transfected with plasmids encoding all viral structural proteins as indicated, as well as expression plasmids for a minigenome, containing a Renilla luciferase reporter gene, and for T7 polymerase. Seventy-two hours after transfection, reporter activity derived from minigenome replication and transcription in these cells (p0) was determined. Supernatant of these cells was then used for infection of Vero E6 target cells previously transfected with plasmids encoding for NP, VP35, VP30, and L (p1). Seventy-two hours postinfection, reporter activity in these p1 cells was determined. The average percentages and the standard deviation of 3 independent experiments are shown. (B) Production of VLPs. 293 cells were transfected with plasmids encoding VP40 (wild-type [WT] or mutant WA, EA, or WEA, as indicated). Forty-eight hours after transfection, the cell lysate and supernatant were collected. The supernatant was cleared of cellular debris and separated into three fractions. Fraction 1 remained untreated, fraction 2 was treated with proteinase K, and fraction 3 was treated with proteinase K and Triton X-100. After 1 h at 37°C, the proteinase was heat inactivated and samples were subjected to SDS-PAGE, Western blotting, and staining using VP40-specific antibodies. (C) Quantification of released VLPs. The amount of VP40 in VLPs and in the cell lysate was quantified using the Odyssey infrared imaging system (Li-Cor) after Western blotting. Depicted is the average ratio of the signal of proteinase K-resistant VP40 in VLPs (B; VLPs + Proteinase K) divided by the VP40 signal in the cell lysate from three independent experiments. Error bars indicate standard deviation.