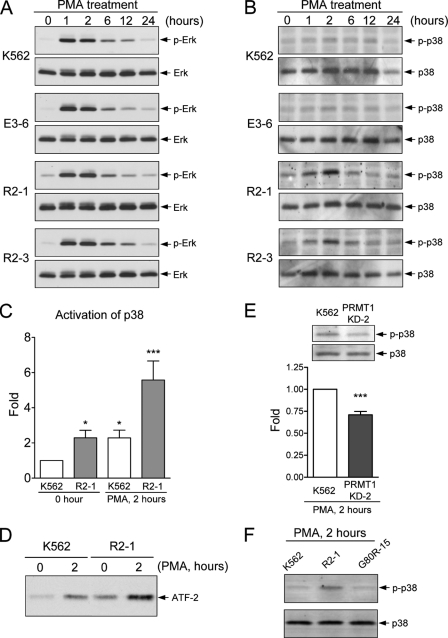
FIGURE 4.
Modulation of the MAPK pathways by PRMT1. Parental, empty control (E3-6), and HA-PRMT1-expressing R2-1 and R2-3 cells were treated with PMA (40 nm), collected, and lysed in RIPA buffer. Activation of ERK (A) and p38 (B) was detected with antibodies against the specific phosphorylated forms. Activation of p38 2 h after PMA treatment was quantified and normalized to the total amount of p38 protein (C). The active form of p38 was immunoprecipitated, and its kinase activity was assayed by phosphorylation of its substrate ATF-2 (D). The kinase activity of p38 was significantly activated in PRMT1-overexpressing cells (C and D). Knockdown of endogenous PRMT1 decreased the activation of p38 (E). Abrogation of PRMT1 enzyme activity (G80R-15 mutant) could no longer promote activation of p38 (F). All experiments were performed at least three times, and data are presented as means ± S.E.; *, p < 0.05; ***, p < 0.005 as compared with K562 parental cells.