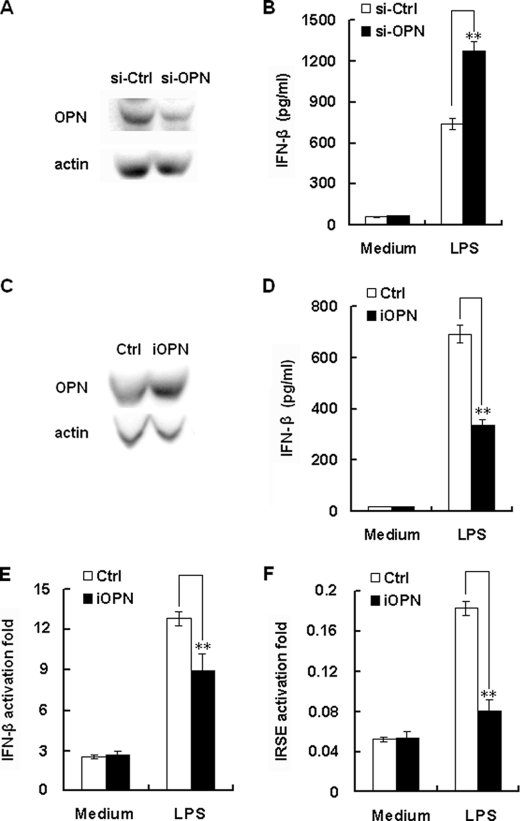
FIGURE 7.
iOPN negatively regulates TLR4-induced IFN-β production. A, expression of endogenous OPN in RAW 264.7 cells was knockdown by si-OPN plasmid. RAW 264.7 cells were stably transfected with si-OPN or si-Ctrl plasmid, and endogenous OPN and actin were detected by Western blot. Similar results were obtained in three independent experiments. B, OPN inhibited LPS-induced production of IFN-β in macrophages. RAW 264.7 cells stably transfected with si-OPN or si-Ctrl plasmid were stimulated with 100 ng/ml LPS for 8 h. IFN-β in the cell culture supernatants were determined by ELISA. The data indicate means ± S.D. of triplicate samples. C, overexpression of iOPN in macrophages. RAW264.7 cells were stably transfected with iOPN expression plasmid, and then confirmed by Western blot. Similar results were obtained in three independent experiments. D, iOPN inhibited LPS-induced production of IFN-β in macrophages. RAW 264.7 cells stably transfected with iOPN or empty pcDNA3.1 construct were stimulated with 100 ng/ml LPS for 8 h. Levels of IFN-β in the cell culture supernatants were determined by ELISA. The data indicate means ± S.D. of triplicate samples. E and F, iOPN inhibited LPS-induced IFN-β or IRSE reporter genes expression in macrophages. RAW 264.7 cells (1.5 × 104/well) in 96-well plates were co-transfected with 100 ng of iOPN or control plasmid, 100 ng IFN-β or IRSE reporter luciferase construct, and 10 ng pTK-Renilla-luciferase. After 24 h, cells were treated with 100 ng/ml LPS for 6 h. Luciferase activity was measured using Dual-Luciferase Reporter Assay system. Data were normalized by Renilla luciferase activity and expressed as fold induction. Data are shown as mean ± S.D. (n = 6) of one representative experiment (**, p < 0.01).