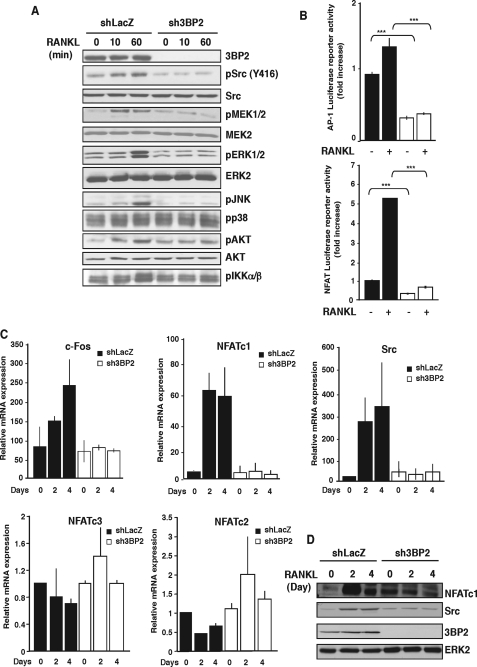
FIGURE 4.
3BP2 knockdown affects multiple signaling pathways in response to RANKL. A, shLacZ and sh3BP2 cells were cultured in the absence of serum for 12 h before stimulation with sRANKL (100 ng/ml) for 0, 10, and 60 min. The cell lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes that were subjected to immunoblot analysis with antibodies against phospho-Src, -ERK1/2, -JNK, -p38, -AKT, -IKKα/β, and -MEK1/2. After stripping, the membrane was reprobed with anti-3BP2, Src, AKT, MEK2, and ERK2. B, shLacZ (black bars) and sh3BP2 (white bars) cells were transfected with AP-1 or NFAT luciferase reporter constructs. 8 h after transfection, the cells were stimulated or not with sRANKL (100 ng/ml). Normalized luciferase activity was determined 24 h after stimulation and expressed as the fold increase relative to basal activities measured in control vector-transfected cells. The results are the means ± S.D. of triplicate determinations. ***, p < 0.001 versus shLacZ. C, cells were stimulated with sRANKL for the indicated times. The expression of c-Fos, Src, NFATc1, NFATc2, and NFATc3 mRNA was determined by real time quantitative PCR. The data are expressed as the means ± S.D. of triplicate determinations and are representative of three independent experiments. D, lysates from cells stimulated as indicated above were subjected to immunoblot analysis with antibodies against NFATc1, Src, and 3BP2. Protein loading was controlled by reprobing the membrane with anti-ERK2.