FIGURE 1.
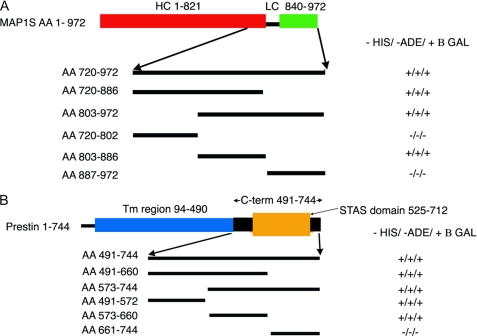
Yeast two-hybrid experiments detect interactions between prestin and MAP1S, which is restricted to specific domains. Initial yeast two-hybrid screening using the C terminus of prestin as bait revealed MAP1S as a binding partner. This clone extended from residues 720 to 972. Subsequently, we used several truncations of these constructs in a yeast two-hybrid assay to clarify the specific domains involved in this interaction. A, initial screening using three equal parts of the protein revealed the interacting domain to lie within the middle fragment (amino acids (AA) 803–886). Subsequent yeast two-hybrid assays (data not shown) using 15–16-amino acid fragments within this region confirmed that the interaction with prestin was mediated by amino acids 819–835 in MAP1S, which lacks predicted secondary structure, contains a large number of proline residues, and lies between the heavy (HC) and light chains (LC) of the protein. Also indicated are the heavy chain (red) and light chain (green) regions of MAP1S relative to the region in MAP1S identified in the yeast two-hybrid screen. B, in a similar approach, we used three equal fragments of the C terminus of prestin in a yeast two-hybrid assay to determine areas that were important in interactions with MAP1S(720–972). We determined that the interacting domains in prestin extended across residues 491–655, which includes parts of the STAS domain and included the entire IVS subdomain within it. We have also shown in schematic form the transmembrane region (blue) of the prestin, its C terminus (black), and the STAS domain (orange) within the C terminus.