FIGURE 1.
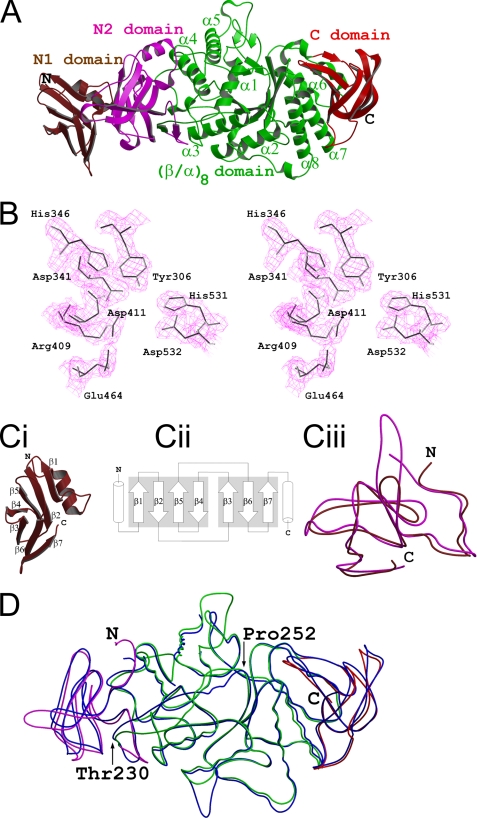
Overall structure. A, the MtbGlgBWT protein contains four domains: the N1 β-sandwich domain (residues 1–105, colored brown), the N2 β-sandwich domain (106–226, magenta), the central (β/α)8 catalytic domain (227–630, green) and the C-terminal β-sandwich (632–731, red). B, electron density around the catalytic pocket formed by the residues that are conserved in the GH13 family (MtbGlgB numbering). The 2Fo − Fc map is contoured at the 1.5 σ level. In C: I, the N-terminal N1 domain has the immunoglobulin sandwich fold. ii, the topological arrangement of the seven β-strands. The two sheets of the sandwich are made by strands β1, β2, β5, and β4 and strands β3, β6, and β7, respectively. Each of the flanking helices comes from the N1 and N2 domains, respectively. iii, superimposition of the N1 (brown) and N2 (magenta) domains of MtbGlgB. The r.m.s.d. is 1.5 Å for 95 Cα atoms. D, superimposition of MtbGlgB (residues 106–731 and domain colors as in A) and ECΔ112GlgB (113–528, blue). The r.m.s.d. is 1.12 Å for 553 Cα atoms. Note the region between residues 230 and 250 (MtbGlgB numbering) is misaligned very much.