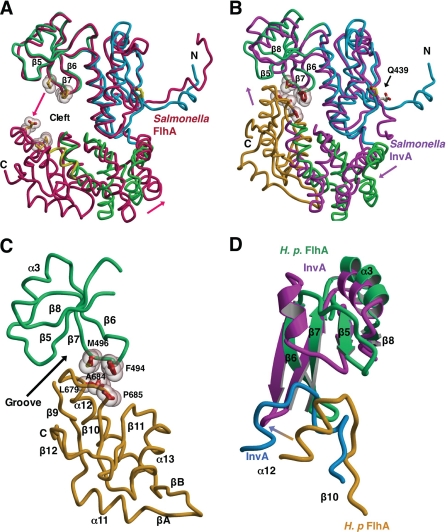
FIGURE 3.
Evidence for a FlhAC conformational change. Structural overlays calculated using lsqkab (45) and drawn with Molscript/Raster3D (49, 50). H. pylori FlhAC drawn as described in the legend to Fig. 1A. A, overlay of H. pylori and Salmonella FlhAC (3A5I, in red) (23) structures based on superposition of the thioredoxin-like and RRM-like domains. The C-domain of H. pylori FlhAC has been omitted for clarity. Arrows depict the relative movement of Salmonella FlhA relative to H. pylori FlhA. Residues in Salmonella FlhA likely to make contact in the presumed closed conformation of the structure are shown as ball-and-stick models with van der Waals surfaces. B, Salmonella InvA (2X49, in magenta) (24) is overlaid onto the structure of H. pylori FlhAC. The C-domain of Salmonella InvA is omitted for clarity. Side chains making van der Waals contact between the RRM-like and C-domains of H. pylori FlhA are drawn as in A and labeled. C, close up of the interface between the RRM-like and C-terminal domains of Hp FlhA. D, comparison of the interface between the RRM-like and C-terminal domains of H. pylori FlhA (in green and gold) and Salmonella InvA (in magenta and blue) (24).