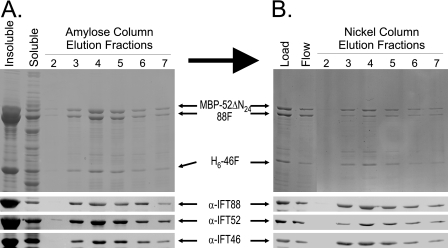
FIGURE 7.
Copurification of H6-46F, MBP-52ΔN24, and IFT88 using tandem affinity chromatography. Upper panels, Coomassie Blue-stained SDS-polyacrylamide gels; lower panels, transfer membranes probed with antibodies directed against IFT88, IFT52, or IFT46. A, MBP affinity purification of coexpressed recombinant H6-46F, MBP-52ΔN24, and untagged IFT88. Soluble bacterial lysate was loaded onto an amylose column. After washing the resin, MBP-52ΔN24 and associated proteins were eluted using 10 mm maltose; fractions 2–7 are shown here. Both H6-46F and the untagged IFT88 coeluted with the MBP-52ΔN24. B, the peak fractions from the MBP affinity chromatography (A) were pooled and further purified using metal (Ni2+) chelate chromatography. Elution of the H6-46F from the Ni2+ resin using imidazole resulted in the coelution of both MBP-52ΔN24 and IFT88, indicating that the three proteins were capable of forming a stable ternary complex.