FIGURE 10.
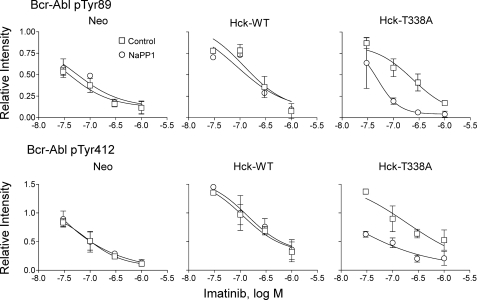
Wild-type Hck or Hck-T338A overexpression increases Bcr-Abl phosphorylation at regulatory tyrosines 89 and 412 in a Hck kinase-dependent manner. K562-Neo, K562-Hck (wild-type (WT)), and K562-Hck-T338A cell populations were plated in 0.5% FBS overnight and treated with the concentrations of imatinib indicated and in the presence or absence of 3 μm NaPP1 for 5 h. Cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with phosphospecific antibodies for Abl pY89 or pY412. Immunoblots were analyzed using the Odyssey Infrared Imaging System. Site-specific phosphotyrosine signal intensities from two independent experiments were normalized to the corresponding levels of Bcr-Abl protein as determined by anti-Abl immunoblotting. The normalized phosphotyrosine band intensities are plotted relative to the ratios obtained from DMSO-treated K562-Neo control cell populations ±S.D. The data points were fitted by nonlinear regression analysis using GraphPad Prizm. Note that Hck expression shifts the imatinib concentration-response curves for both phosphorylation sites upward and to the right, indicative of enhanced phosphorylation and reduced drug sensitivity. These shifts are completely and exclusively reversed in Hck-T338A cells after treatment with NaPP1. Images of the immunoblots used to generate these curves, as well as additional data for Bcr-Abl Tyr-245 and Tyr-177 are shown in supplemental Fig. S3.