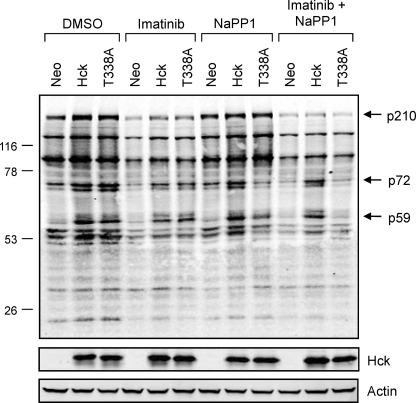
FIGURE 8.
Wild-type Hck or Hck-T338A overexpression enhances tyrosine phosphorylation of several proteins in K562 cells without changing the overall tyrosine phosphoprotein banding pattern. K562 cells were infected with recombinant retroviruses carrying a neomycin selection marker (Neo), wild-type Hck or Hck-T338A and selected with G418. The resulting cell populations were plated in 0.5% FBS overnight and treated with the DMSO carrier solvent, imatinib (1 μm), NaPP1 (3 μm), or imatinib plus NaPP1 for 5 h. Cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and probed with anti-phosphotyrosine (top) and anti-Hck antibodies. Immunoreactive proteins were simultaneously detected using the Odyssey Infrared Imaging System (LI-COR). As a loading control, replicate blots were also probed with an anti-actin antibody. Using the Odyssey 3.0 software, the position of each molecular weight marker was used to estimate the molecular weights of each tyrosine phosphoprotein. The positions of the three bands showing the most prominent changes in phosphotyrosine content after Hck expression are indicated (p210, p72, and p59). This entire experiment was repeated three times from independently derived cell populations and produced comparable results in each case.