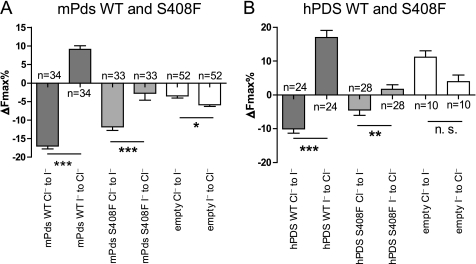
FIGURE 2.
The S408F mutation causes a significant reduction of the related anion transport tested for the mouse pendrin (A), as well as for the human pendrin (B). A, change of the fluorescence signal (expressed as maximal fluorescence variations, ΔFmax%) after the Cl− to I− or I− to Cl− substitutions (for details, see “Experimental Procedures”) in HEK 293 Phoenix cells expressing only the EYFP protein (empty) or the EYFP protein and mouse wild-type pendrin (mPds WT) or mutated mouse Pds (mPds S408F). The numbers of the experiments are given (n). Error bars indicate the S.E. Statistical analysis: ***, p < 0.001; *, = p < 0.05; paired Student's t test. Error bars indicate S.E. B, change of the fluorescence signal (expressed as maximal fluorescence variations, ΔFmax%) after the Cl− to I− or I− to Cl− substitutions (for details, see “Experimental Procedures”) in HEK 293 Phoenix cells expressing only the EYFP protein (empty) or the EYFP protein and human wild-type PDS (hPDS WT) or mutated human PDS (hPDS S408F). The numbers of the experiments are given (n). Error bars indicate the S.E. Statistical analysis: ***, = p < 0.001; **, = p < 0.01; n. s. = not significant; paired Student's t test. Error bars indicate S.E. The results of the analysis of variance test with Bonferroni's multiple comparison post-test are reported under “Results.” n represents the number of cells.