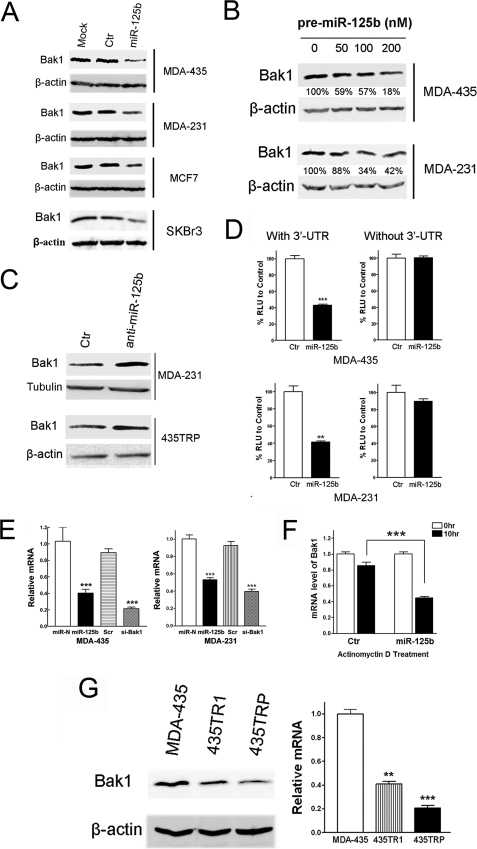
FIGURE 4.
Bak1 is a direct target of miR-125b. A, breast cancer cells, MDA-435, MDA-231, MCF7, and SKBr3 were transfected with 100 nm pre-miR-negative (Ctr) or pre-miR-125b. Twenty-four hours after transfection, cell lysates were prepared for Western blotting with an antibody against Bak1, and β-actin was used as a loading control. B, increasing concentrations (0, 50, 100, 200 nm) of pre-miR-125b were transfected into MDA-435 and MDA-231 cells, and then the cells were collected for Western blotting. The membranes were incubated with an antibody against Bak1. β-Actin was used as a loading control. The gray density was quantified using Scion image software and normalized to the β-actin. C, MDA-231 and 435TRP cells were transfected with 100 nm anti-miR-negative (Ctr) or anti-miR-125b, respectively. Twenty-four hours after transfection, cell lysates were extracted for Western blotting with an antibody against Bak1. β-Actin was used as a loading control. D, MDA-435 cells (top) and MDA-231 cells (bottom) were co-transfected with luciferase reporter plasmids with or without 3′-UTR of Bak1, pre-miR-125, or pre-miR-negative (Ctr) by using Lipofectamine 2000 reagent. Thirty-six hours post-transfection, cells were harvested and lysed with passive lysis buffer. Luciferase activity was measured by using a dual luciferase reporter assay. The pRL-TK vector was used as an internal control. The results were expressed as relative luciferase activity (firefly LUC/Renilla LUC). RLU, relative luciferase units. E, MDA-435 cells (left) and MDA-231 cells (right) were transfected with pre-miR-negative, pre-miR-125b, Scramble siRNA, or siRNA to Bak1. Twenty-four hours after transfection, the cells were collected, and Bak1 mRNA levels were measured by qRT-PCR. The relative Bak1 mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase siRNA to Bak1 served as a positive control. F, MDA-435 cells were transfected with 100 nm pre-miR-125b or pre-miR-negative. After 12 h, cells were treated with actinomycin D (5 μg/ml) 10 h. The stability of endogenous Bak1 mRNA was determined by real-time RT-PCR at the indicated time. G, cell lysate or cDNA was prepared from 435TR1, 435TRP, and their parental MDA-435 cells, and then the protein or cDNAs were subjected to Western blotting or qRT-PCR assays for detection of Bak1 protein (left) or mRNA expression (right). For qRT-PCR assay, relative mRNA expression of Bak1 was shown in the bar diagram from three independent experiments, and glyceraldehyde-3-phosphate dehydrogenase was used as a loading control. Columns, mean of three independent experiments; bars, S.E. *, p < 0.05, **, p < 0.01, ***, p < 0.001.