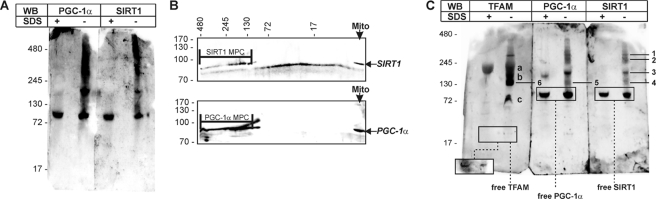
FIGURE 6.
Identification of mitochondrial multiprotein complexes by BN-PAGE in liver purified mitochondria. A, mitochondrial proteins were extracted from liver purified mitochondria (mito) using a lysis buffer maintaining native interaction in multiprotein complexes. Ten μg of proteins were loaded on blue native gel. The same samples were boiled in the presence of SDS and loaded in parallel with the respective native sample. Western blot (WB) analysis using rabbit Santa Cruz Biotechnology anti-SIRT1 or anti-PGC-1α was then performed. B, after BN-PAGE, a second SDS-PAGE dimension was carried out followed by Western blot analysis using rabbit Santa Cruz Biotechnology anti-PGC-1α or anti-SIRT1. MPC, multiprotein complexes. C, 10 μg of proteins were loaded on blue native gel, and Western blot using a rabbit anti-TFAM was carried out. 1, multiprotein complex containing TFAM, PGC-1α, and SIRT1; 2 and 3, multiprotein complexes containing PGC-1α and SIRT1; 4, multiprotein complex containing TFAM, and SIRT1; 5 multiprotein complex containing TFAM and PGC-1α; 6, multiprotein complex containing TFAM-PGC-1α and TFAM-SIRT1; a–c, other TFAM multiprotein complexes. To visualize free TFAM protein, a major exposure was mandatory due to the lower cross-reactivity of TFAM antibody against native TFAM with respect to TFAM engaged in multiprotein complexes (see inset in TFAM immunoblot). Immunoblots reported are from one experiment representative of four that gave similar results.