FIGURE 2.
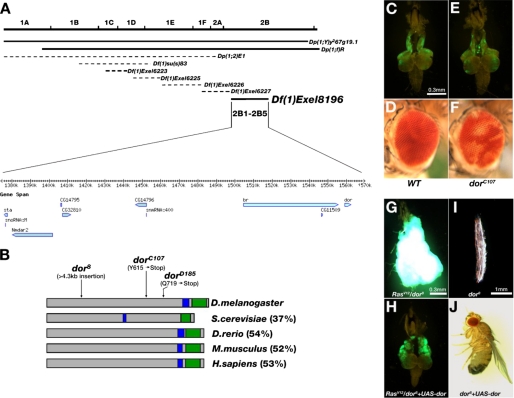
deep orange behaves as a metastasis suppressor. A, genetic mapping using a series of duplication and deficiency lines located the dorC107 mutation in the chromosome region of 2B1-2B5. Solid lines indicate the duplications and deficiencies that rescued or failed to complement with the mutation, respectively. Dotted lines indicate the duplications or deficiencies that failed to rescue or were able to complement the mutation, respectively. The candidate genes located within this region are shown. B, illustration of the Dor protein and its homologues in several species. The coiled-coil domain and the RING-H2 domain are indicated by blue and green boxes, respectively. The percentages of overall similarity between Dor and its homologues are indicated. The molecular lesions of the three dor alleles are also indicated. D.melanogaster, Drosophila melanogaster; S.cerevisiae, Saccharomyces cerevisiae; D.rerio, Danio rerio; M.musculus, Mus musculus; H.sapiens, Homo sapiens. C, E, G, and H, fluorescence images of dissected cephalic complexes showing GFP-labeled homozygous cell clones of indicated relevant genotypes. D and F, micrographs of mosaic compound eyes containing homozygous WT (D) or dorC107 (F) clones labeled by the w mutation. I and J, microscopy images of the three-instar larva (I) and the adult fly (J) with indicated genotypes. Clones homozygous for dorC107 (E) do not exhibit any growth advantage when compared with WT (C). Note the severe pigmentation loss in dorC107 clones (F) when compared with WT (D). G–J, the expression of full-length wild type dor cDNA can fully rescue dor8-caused phenotypes. The excess growth and metastasis of RasV12/dor8 tumors (G) are completely blocked by specific expression of UAS-dor in these tumor clones (H). The image of a viable adult fly indicates that the lethality of dor8 allele (I) is rescued by ubiquitous expression of UAS-dor (J). C and E are in the same magnification. G and H are in the same magnification.