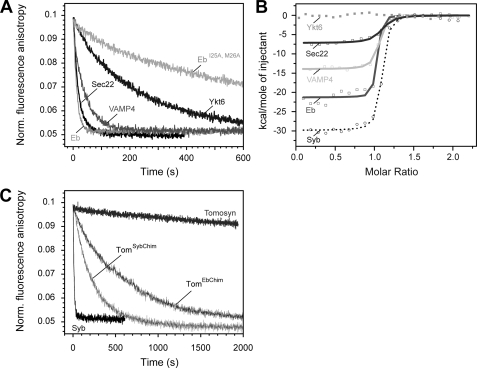
FIGURE 5.
Different abilities of synaptobrevin homologs to bind to the ΔN complex. A, the rate of displacement of Syb49–96 from the ΔN complex monitored by fluorescence anisotropy. As in Fig. 2C, ∼100 nm labeled ΔN complex (SyxH3·SNAP-25·Syb49–96C79Alexa488) was incubated with the SNARE domain of different synaptobrevin homologs (500 nm) (12, 13). A depiction of the different constructs used is shown in supplemental Fig. S3. Endobrevin and Sec22 were able to quickly displace Syb49–96, whereas VAMP4 was slightly slower. Possibly the somewhat reduced binding efficiency of VAMP4 compared with synaptobrevin 2 is due to an exchange of the conserved isoleucine to a valine in the site preceding layer −3 in this R.III-SNARE subtype, which is specific to animals. Only slow displacement was observed for Ykt6, whereas tomosyn was unable to displace the fragment (C). B, interaction of the SNARE domain of different synaptobrevin homologs and the ΔN complex measured by ITC. Titrations of VAMP4 and Eb (15 μm) into a purified ΔN complex (1.7 μm). Sec22 (40 μm) was titrated into 4.25 μm ΔN complex. Upon titration of Ykt6 or tomosyn into the ΔN complex, no binding was detected. For comparison, the titration of Syb1–96 into the ΔN complex is shown again (12). C, chimeras of tomosyn containing a functional trigger site are able to displace the Syb49–96 from the ΔN complex. As in A, displacement was monitored by fluorescence anisotropy of 100 nm labeled ΔN complex (SyxH3·SNAP-25·Syb49–96C79Alexa488) after the addition of 500 nm of the tomosyn chimeras. For comparison, the reaction is shown in the presence of Syb1–96 and tomosyn.