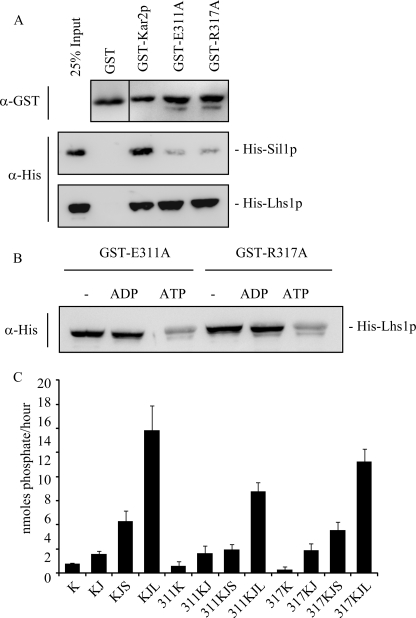
FIGURE 5.
Mutation of residues Glu-311 and Arg-317 affects Sil1p binding and function. A, ability of Kar2p E311A and R317A to bind to His-Sil1p and His-Lhs1p was assayed in a GST pulldown alongside wild-type Kar2p. Binding of the His-tagged proteins was visualized by Western blotting. B, effect of nucleotide on the interaction between His-Lhs1p and the Kar2p mutants was assayed by repeating the binding experiments in the presence of 2 mm ADP or ATP. C, steady state ATPase activity of Kar2p was measured by a colorimetric assay at A640 nm, and the amount of phosphate released was calculated using a standard curve. Kar2p (K) or the Kar2p mutants (311K or 317K) were incubated in the presence of 0.8 μm GST-J domain of Sec63 (J) and with His-Sil1p (S) or His-Lhs1p (L) (as indicated) for 1 h at 25 °C in the presence of 2 mm ATP.