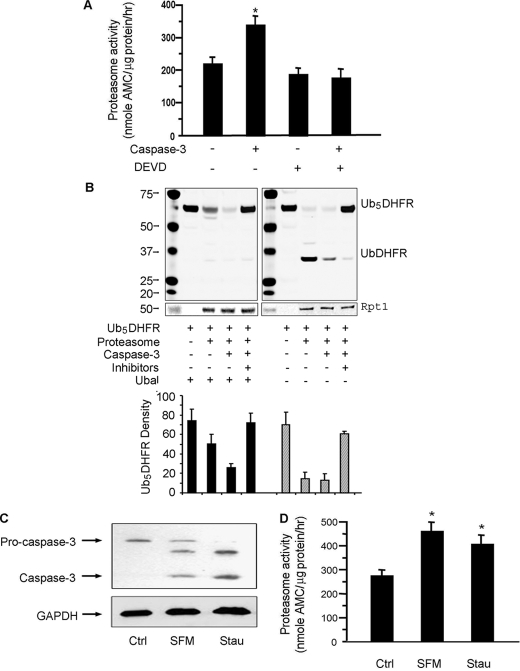
FIGURE 1.
Caspase-3 stimulates proteasome activity in C2C12 myotubes. A, proteasomes isolated from myotubes were incubated with 100 nm recombinant caspase-3 with or without its inhibitor (DEVD-CHO). Proteasome activity in equal amounts of proteasome protein was measured using the fluorogenic peptide, LLVY-AMC. The results are the mean ± S.E. of replicate assays from four proteasome preparations (*, p < 0.05 versus proteasomes untreated by caspase-3). B, activity of proteasomes from A was also evaluated by measuring degradation of the ubiquitin-conjugated substrate, Ub5DHFR. Proteasomes isolated from myotubes were incubated with or without caspase-3 for 1 h; in the indicated mixtures, MG-132 plus lactacystin were used to block proteasome activity. In the left panel, ubiquitin aldehyde (Ubal) was added to all reactions to inhibit deubiquitination. In the right panel, Ubal was not added. An anti-His antibody was used in the Western blots and an antibody against Rpt1 was used to assess the loading of proteasomes. The bar graph underneath is the density analysis of Ub5DHFR based on three different Western blots. (*, p < 0.05 versus no proteasome added). C, myotubes were incubated in serum-free medium (SFM) or with 50 nm staurosporine (Stau). Pro-caspase and activated caspase-3 were assessed by Western blots. D, proteasome activity in myotubes incubated in SFM or treated with staurosporine was measured as described in A (n = 4; *, p < 0.05 versus untreated myotubes (Ctrl)).