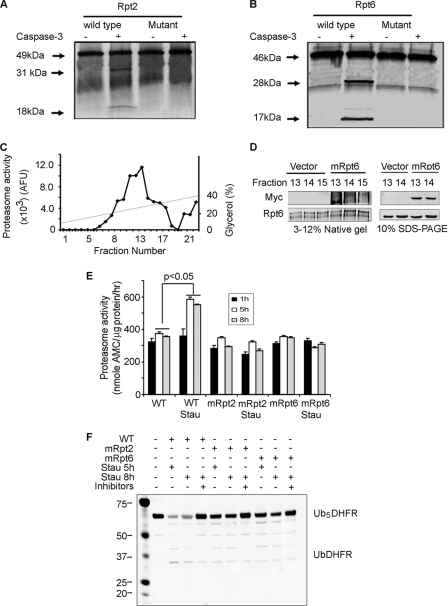
FIGURE 3.
In Rpt2 and Rpt6, mutation of caspase-3 cleavage sites suppresses staurosporine-induced proteasome activity in C2C12 myotubes. A and B, recombinant caspase-3 cleaved l-[35S]methionine- labeled, in vitro translated Rpt2 or Rpt6. Mutation of the caspase-3 cleavage site in Rpt2 and Rpt6 abolished caspase-3-induced cleavage. C, C2C12 cell lysates were subjected to glycerol density gradient centrifugation and proteasome fractions were assayed for proteasome activity using LLVY-AMC. D, proteasomes from wild-type cells and cells expressing mutant Rpt6 (mRpt6) were separated by glycerol density centrifugation and fractions 13–15 were subjected to native PAGE (left) or SDS-PAGE (right). Using anti-Myc or anti-Rpt6 antibodies, the mutant Rpt6 was found to be present in 26 S proteasomes by detection of Myc. E, wild-type cells and cells stably transfected to express mutated Rpt2 (mRpt2) or Rpt6 (mRpt6) were treated with or without 50 nm staurosporine for 1, 5, or 8 h. Proteasomes isolated from these cells were used to measure proteasome activity using LLVY-AMC as a substrate (n = 3; *, p < 0.05 versus proteasomes subunits not exposed to staurosporine). F, Proteasomes from myotubes treated with staurosporine for 5 or 8 h (see E) were incubated with Ub5DHFR in the presence of Ubal. The anti-His antibody (see Fig. 1) was used to detect His-tagged ubiquitins conjugated to DHFR. The staurosporine-induced increase in proteasome activity was blocked in cells expressing the mutated subunits (E and F).