Abstract
Purpose
The present study evaluated the effects of fatty acids commonly present in cosmetic and topical formulations on permeation enhancement across human epidermal membrane (HEM) lipoidal pathway when the fatty acids saturated the SC lipid domain without cosolvents (Emax).
Methods
HEM was treated with neat fatty acids or fatty acid suspensions to determine Emax. A volatile solvent system was used to deposit fatty acids on HEM surface to compare fatty acid enhancer efficiency in topical volatile formulations with Emax. To elucidate permeation enhancement mechanism(s), estradiol (E2β) uptake into fatty acid-treated SC lipid domain was determined.
Results
Emax of fatty acids was shown to increase with their octanol solubilities and decrease with their lipophilicities, similar to our previous findings with other enhancers. Emax of solid fatty acids was shown to depend on their melting points, an important parameter to the effectiveness of the enhancers. The E2β uptake results suggest that enhancer-induced permeation enhancement across HEM is related to enhanced permeant partitioning into the SC lipid domain.
Conclusions
The results suggest Emax as a model for studying the permeation enhancement effect of the fatty acids and their structure enhancement relationship.
Keywords: chemical penetration enhancers, corticosterone, Emax, estradiol, fatty acids, skin
INTRODUCTION
Transdermal drug delivery possesses a number of advantages but the relative low permeability of skin is the major impediment (1,2). Approximately 10–20 drugs or drug combinations are efficiently delivered via skin, including estrogen, scopolamine, nitroglycerine and nicotine (3). To overcome the diffusional barrier property of skin, percutaneous penetration enhancers are used (1,4). Chemical penetration enhancers used in transdermal products are usually pharmacologically inactive, capable of partitioning into the stratum corneum (SC), modifying its properties and thus enhancing drug permeation (5). Chemical enhancers are thought to enhance permeation through a number of mechanisms, mainly by the disruption of the ordered SC lipid structure and sometimes by the alteration of the SC keratinocytes (protein conformational change) (6–8).
For several decades, some chemical enhancer studies have focused on screening of enhancers in topical or transdermal formulations (9). Other studies have attempted to understand the mechanisms of chemical enhancers and to evaluate enhancer efficiency, thus facilitating and optimizing chemical enhancer selection (6,10). The permeation enhancement effect Emax was recently defined as the permeation enhancement induced by an enhancer on the lipoidal transport pathway of human epidermal membrane (HEM) when the enhancer thermodynamic activity approached unity in equilibrium with HEM (11); note that the symbol Emax in the present study should not be confused with those in other studies such as that for enzymatic maximum velocity. The Emax of a number of chemical enhancers has been previously determined using a direct approach to overcome the difficulties associated with studying lipophilic enhancers in aqueous media in the absence of cosolvents and solubilizing agents. This direct approach allows the elucidation of enhancer sole mechanism of action without potential synergistic effects (12) from cosolvents and solubilizing agents. The study of the sole impact of enhancers on transdermal delivery can also provide useful information in the development of formulations composed of a volatile carrier system (13), in which the enhancer deposited on skin would exclusively impact skin permeation.
The definition of Emax and the associated findings in recent studies have contributed to the understanding of chemical enhancer mechanisms of action (11,14). In these studies, it was found that (a) the interaction between the studied enhancers and SC lipid domain is non-specific and relatively independent of the alkyl chain length and polar head groups of the enhancers, (b) the efficiencies of the enhancers are related to their solubilities in the SC lipid domain, (c) the enhancers enhance permeation by enhancing permeant partitioning into the SC lipid domain, (d) the enhancers result in an increase in the fluidity of the SC lipid domain, and (e) Emax of the enhancers decreases with increasing enhancer lipophilicities (enhancer Koct).
Fatty acids have been shown to interact with the SC lipids, and a number of fatty acids have been identified as skin permeation enhancers (15,16). The effects of fatty acids as permeation enhancers have been shown to be dependent on their structure, alkyl chain length, and degree of saturation (17). Unsaturated fatty acids have been shown to promote higher magnitudes of permeation enhancement across skin when compared to saturated fatty acids of the same chain length. This has been attributed to the higher disrupting nature of the kinked chain of these fatty acids that would result in a higher magnitude of lipid disruption (18–20).
Many fatty acids are generally recognized as safe (GRAS listed) and are approved by the FDA as inactive ingredients in a number of products. For example, oleic acid is a constituent of an estradiol transdermal formulation Vivelle® acting as a permeation enhancer in the presence of propylene glycol. The lipophilic nature of moderate to long chain fatty acids has led to the predominant use of cosolvents in the study of fatty acid permeation enhancement effects. It has been demonstrated that fatty acid permeation enhancement is highly dependent on the nature of the cosolvent used. In a previous study, benzyl alcohol was used as a carrier system for a number of fatty acids (oleic, palmitoleic and linoleic acids) used in a 14 h pre-treatment process. It was shown that oleic acid had the lowest magnitude of permeation enhancement (enhancement ratio of 2.4 at 20% oleic acid) of methylparabene, while palmitoleic acid demonstrated the highest enhancement effect (enhancement ratio of 13.4 at 20% palmitoleic acid) (21). In a similar study, propylene glycol was used as a cosolvent, and the effects of oleic acid and palmitoleic acid on the permeation of indomethacin were similar and resulted in an approximately ten-fold permeation enhancement across skin (22). Fatty acids therefore exhibit synergistic enhancement effects with cosolvents present in transdermal formulations, and the sole permeation enhancement effects of many fatty acids are not known.
The present study was a continuation of our effort to understand the mechanism(s) of action of chemical permeation enhancers and to evaluate the Emax approach in the study of enhancer efficiency. The permeation enhancers evaluated in the present study were fatty acids commonly present in pharmaceutical, cosmetic, and food products. The objectives of the present study were to determine (a) the effectiveness of fatty acids based on their maximum enhancement effect, Emax, (b) the relationship between fatty acid Emax and lipophilicity and if fatty acids follow the previously observed enhancer Emax vs. enhancer lipophilicity relationship, (c) the effectiveness of using silicone elastomer to estimate fatty acid Emax, (d) the flux enhancement induced by fatty acids deposited from a volatile solvent system and its relationship to Emax, and (e) the effect of fatty acids on the diffusivity and partitioning of a model permeant in HEM SC. Table I presents a summary of the fatty acids used in the present study, their abbreviations, and the nature of the formulations in which they are used.
Table I.
A Summary of the Fatty Acids Used in the Present Study, Their Chemical Formulas and a Summary of Commercial Products Containing Them
| Fatty Acid | Chemical Formula | Product Information/Uses (Formulation Type) |
|---|---|---|
| Decanoic acid (DCA) | CH3(CH2)8COOH | Used in food processing: sanitizing agent, coating for fruits and vegetables |
| Undecanoic acid (UDA) | CH3(CH2)9COOH | NA |
| Lauric acid (LRA) | CH3(CH2)10COOH | Commonly used in cosmetic products such as hair color and bleaching and body wash/cleanser |
| Tridecanoic acid (TDA) | CH3(CH2)11COOH | Usage of up to 8% in fragrance concentrate |
| Myristic acid (MA) | CH3(CH2)12COOH | Commonly used in cosmetic products such as facial cleanser and facial moisturizer/treatment |
| Pentadecanoic acid (PDA) | CH3(CH2)13COOH | Used in baked goods, milk products, and seasonings and foods |
| Palmitic acid (PA) | CH3(CH2)14COOH | Commonly used in cosmetic products such as mascara and shaving cream |
| Stearic acid (STA) | CH3(CH2)16COOH | Commonly used in cosmetic products such as moisturizer and sunscreen |
| Oleic acid (OL) | CH3(CH2)7CH=CH(CH2)7COOH | Commonly used in cosmetic products such as hair color and shampoo |
| Linoleic acid (LA) | CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH | Commonly used in cosmetic products such as facial moisturizer/treatment and sunscreen |
| Ricinoleic acid (RCA) | CH3(CH2)5CH(OH)CH2CH=CH(CH2)7COOH | Used in cosmetic products such as baby shampoo and conditioner |
EXPERIMENTAL METHODS
Materials
3H-Corticosterone (CS) was purchased from Perkin Elmer Life and Analytical Sciences (Boston, MA) at purity >97%. Oleic acid was purchased from Fisher Chemicals (Pittsburgh, PA) at purity > 95%. Stearic acid, palmitic acid, lauric acid, myristic acid, linoleic acid and sodium azide (NaN3) were purchased at purities ≥98% from Acrōs Organics (Morris Plains, NJ). Trypsin from bovine pancreas was purchased from MP biomedical (Santa Ana, CA). Estradiol (E2β) micronized USP was purchased from Letco (Decatur, AL) and Spectrum Chemical and Laboratory Supplies (Gardena, CA) at purity≥97%. Decanoic acid, undecanoic acid, tridecanoic acid, ricinoleic acid and pentadecanoic acid at purities≥98% and corticosterone at purity>92% were purchased from Sigma-Aldrich, Co. (Saint Louis, MO). Phosphate-buffered saline (PBS: 0.01 M phosphate buffer, 0.0027 M potassium chloride, 0.137 M sodium chloride) at pH 7.4 was prepared by dissolving phosphate buffer tablets in distilled de-ionized water and preserved using 0.02% sodium azide (NaN3). Silicone elastomer components (MED-6033) were purchased from NuSil Technology (Carpinteria, CA). The diffusion cells used in the transport experiments consisted of two half cells, each cell with a volume capacity of approximately 2 ml and diffusional area of approximately 0.8 cm2. Posterior torso split-thickness frozen cadaver skin was obtained from the New York Firefighters Skin Bank (New York, NY). Frozen human cadaver skin was previously found to have transport properties that differed only slightly from those of allograft skin (23). Human epidermal membrane (HEM), consisting of the SC and the viable epidermis, was prepared by the removal of the dermis via heat separation of the split-thickness skin and stored at −20°C for later use (11).
HEM PBS Control Transport Experiments
Passive transport across HEM was conducted in a side-by-side diffusion cell as described previously (11). Briefly, HEM was sandwiched between two Millipore filters (Durapore membrane filters, 0.22 μm pore size), one on each side, and two rubber gaskets in a side-by-side diffusion cell. To prevent leakage from cells, the interface of the two diffusion cell halves was sealed using Parafilm. In a circulating water bath kept at 37°C, HEM was allowed to equilibrate in PBS (2 ml in both the receiver and donor chamber). HEM electric resistance was determined before and after equilibration using the same method described previously (11). HEM samples deemed of suitable integrity, with initial electrical resistance≥15 kΩcm2, were used in the present study. 3H-CS (≈ 0.1 μCi) was pipetted into the donor chamber, and passive transport was conducted under stirring for at least 4 times the transport lag time. Samples from both chambers were taken (2 ml samples from the receiver chamber with replacement of the same volume of fresh solution and 10 μl samples from the donor) at predetermined time intervals. The samples were mixed with 10 ml of scintillation cocktail (Ultima Gold, Boston, MA) and analyzed using a liquid scintillation counter (Beckman Coulter LS 6500 Multipurpose Scintillation Counter, Fullerton, CA).
The permeability coefficient of CS (24) across HEM was calculated by
| (1) |
where CD is the model permeant donor concentration, A is the diffusional area of the side-by-side diffusion cell, and dQ/dt is the slope of the plot of the receiver chamber cumulative amount of permeant versus time in the steady state region.
HEM Transport Experiments: Enhancer (Fatty Acid) Studies to Determine Emax
Solid Fatty Acids
HEM was mounted in a side-by-side diffusion cell as stated under “HEM PBS Control Transport Experiments.” Following HEM hydration, PBS was removed from the diffusion cell chambers, and 2 ml of solid fatty acid (DCA, UDA, LRA, TDA, MA, PDA, PA, and STA) suspension (5–10 mg of fatty acid in 10 ml PBS and stirred for 24–48 h) were pipetted in both chambers. HEM was maintained under well-stirred conditions at 37°C for 5–6 h; both chamber contents were replaced with fresh fatty acid suspension at least 3 times during the enhancer equilibration step. Fatty acid suspension was used to treat both sides of HEM to allow a symmetric distribution of fatty acid within SC, thus avoiding a complex mechanistic interpretation of the data as a result of a fatty acid concentration gradient in SC when only one side of HEM (e.g., the SC side) was equilibrated with the fatty acid. Following fatty acid equilibration with HEM, fatty acid suspension was completely removed from both chambers. PBS was used to rinse both chambers at least 5–6 times. PBS saturated with fatty acid prepared by fresh fatty acid suspension and centrifugation (fatty acid was centrifuged and then the clear supernatant was collected) was used as the transport vehicle, and 2 ml of which were pipetted into both chambers. 3H-CS was added to the donor chamber, and passive transport experiment was conducted as described in “HEM PBS Control Transport Experiments,” except that the transport vehicle was PBS saturated with fatty acids.
Liquid Fatty Acids
A similar approach of direct enhancer treatment previously described was adapted in the present study (11). Briefly, 20 ml of LA or RCA saturated with PBS were pipetted into a Petri dish. Fully hydrated HEM was then placed in the Petri dish and allowed to equilibrate with the fatty acids for 20 min. HEM was then removed and rinsed with PBS three times and patted with a Kimwipe between each rinse. Fatty-acid equilibrated HEM was then mounted in a side-by-side diffusion cell, and fatty acid saturated PBS solution was pipetted into both chambers. Passive transport experiments was conducted as stated above.
Corticosterone Solubility Analysis
Prior to the solubility study, PBS saturated with fatty acid was analyzed using Malvern Zetasizer® Nano (Malvern Instruments Ltd, United Kingdom) to check for the presence of micelles. In the solubility study, precisely weighed 5 mg of CS was added to a pyrex culture tube containing 2 ml fatty acid-saturated PBS. The culture tubes were then left in a shaking waterbath at 37 °C for 72 h, after which the tubes were subjected to 15–20 min centrifugation at 3400 rpm (Fisher Centrific model 228). The supernatant was then collected and filtered through a 0.45 μm Millipore MF™ Membrane filter (Bioscience, Life Science Products, Dayton, OH). The filtered supernatant (the first portion of which was discarded) was diluted and analyzed using HPLC.
Emax Calculation and Theory
Previously, Emax of an enhancer was determined by the equilibration of HEM with a pure enhancer or an enhancer-saturated PBS solution (11). This was based on the assumption that the enhancer at saturation in a solution had thermodynamic activity approaching that of its pure state. In the present study of liquid fatty acids, the method of direct pure enhancer treatment was used. For the solid fatty acids, the use of a fatty acid suspension for the treatment of HEM would prevent fatty acid depletion from the equilibrating aqueous solution and introduce fatty acid at a thermodynamic activity approaching that of its pure state. In both liquid and solid fatty acid enhancer cases, the donor and receiver solutions were fatty acid-saturated PBS during CS transport. Emax was calculated using the ratio of the permeability coefficients of CS in the presence and absence of the fatty acids with HEM samples from the same skin donor:
| (2) |
where PL,enhancer is the permeability coefficient of the lipoidal pathway when the thermodynamic activity of the enhancer in equilibrium with HEM approaches its pure state, PL,PBS is the permeability coefficient of the lipoidal pathway of control HEM in PBS. Senhancer is the solubility of CS in the fatty acid-saturated PBS solution, and SPBS is the solubility of CS in PBS. The ratio of the solubility is used to correct for any changes in CS thermodynamic activity in the fatty acid-saturated PBS from that in PBS.
Flux Enhancement with Fatty Acid Deposition from Volatile Solvent (Enhancer/Ethanol Treatment) and Duration of Flux-Enhancing Effect
HEM samples were hydrated for 2–4 h in 20 ml PBS in a Petri dish and then lifted using a filter paper, and the HEM and filter paper composite was placed on a PBS-wetted support as described previously (14). After that, the HEM samples were treated with 0.35 ml of 8% w/v and 8% v/v enhancer in ethanol for the solid enhancers (DCA, UDA, LRA, TDA, MA, PDA, and PA) and the liquid enhancers (OL, LA, and RCA), respectively. For STA, due to its relatively low solubility in ethanol, 3% w/v STA (instead of 8%) was used. The 3–8% enhancers were deemed sufficient to saturate the SC lipids, where the amount of enhancer deposited on HEM was much greater than the total volume of intercellular lipid in HEM SC used in the study (≈ 20% of the SC volume). HEM samples were then allowed to equilibrate on the PBS-wetted support for 5–6 h for the solid enhancers and for 20 mins for the liquid enhancers. After equilibration, HEM was rinsed 3 times with PBS and patted with Kimwipes between each rinse. HEM was then mounted in a side-by-side diffusion cell and allowed to equilibrate with stirring at 37°C for 2 h, allowing the redistribution of fatty acids in SC. Both chambers were then replaced with fresh PBS, and passive transport was conducted as described in “HEM PBS Control Transport Experiments.” HEM treated with 0.35 ml ethanol was used as the control.
Calculation of Enhancement Factor on CS Permeation
The enhancement factor (E) of CS transport was determined by the ratio of the permeability coefficients of the permeant of fatty acid/ethanol-treated HEM to that of ethanol-treated HEM control (14):
| (3) |
where PL,enhancer/ethanol is the permeability coefficient of HEM lipoidal pathway after enhancer/ethanol treatment, and PL, control is the permeability coefficient of the lipoidal pathway of ethanol-treated HEM (control) from the same skin donor.
Estradiol (E2β) Partitioning into Human Stratum Corneum Lipid Domain
SC was isolated from HEM, from which n-hexane-treated and delipidized SC were obtained using a method previously described (14). The dry and wet weights of both n-hexane-treated and delipidized SC were carefully determined. To hydrate SC (n-hexane-treated or delipidized SC), SC samples were placed in scintillation vials containing 10 ml of PBS for 4 h. After hydration, n-hexane-treated or delipidized SC was allowed to float on PBS in a Petri dish and then lifted using filter paper, and the SC and filter paper composite was placed on PBS-wetted cotton support. SC (n-hexane-treated or delipidized) was then treated with fatty acids as stated under “Flux-Enhancement with Fatty Acid Deposition from Volatile Solvent (Enhancer/Ethanol Treatment) and Duration of Flux-Enhancing Effect.” SC (n-hexane-treated or delipidized) was then placed in scintillation vials filled with 20 ml of E2β suspension (1.5 mg/ml E2β in PBS) (14). The scintillation vials were then sealed with Parafilm and kept in a shaker at 37°C for 12 h, after which the SC (n-hexane-treated or delipidized) was removed and rinsed with deionized water three times and patted using Kimwipes after each rinse. The SC (n-hexane-treated or delipidized) was then extracted with 5 ml of absolute HPLC-grade ethanol in scintillation vials at room temperature on a low-speed shaker for 48 h. The ethanol solutions were centrifuged at 3400 rpm (Fisher Centrific Model 228) in Pyrex culture tubes for 30 mins. The clear supernatant was filtered through a 0.45-μm Millipore filter (MF™ membrane, Bioscience, Life Science Products) discarding the first portion, and analyzed for E2β. E2β uptake into n-hexane-treated and delipidized SC was then calculated. Ethanol-treated SC (n-hexane-treated and delipidized) was used as the control.
E2β Uptake Calculation
The equilibrium uptake amount of E2β (14) was calculated by
| (4) |
where Ecorrected is the amount of E2β uptake into the n-hexane-treated SC (or delipidized SC) expressed here in micromoles of E2β uptake per milligrams of dry n-hexane-treated SC (or delipidized SC), Eextracted is the amount of E2β extracted from the n-hexane-treated SC (or delipidized SC), Wdry is the dry n-hexane-treated SC weight (or dry delipidized SC weight), Wwet is the wet weight of n-hexane-treated SC (or the wet delipidized SC weight), and Saq is the aqueous solubility of E2β. The second term in the equation was used to correct for the E2β uptake in the aqueous compartment within the SC.
Silicone Elastomer Enhancer Uptake
The study of silicone elastomer enhancer uptake followed a similar experimental approach described in a previous study (11). Briefly, silicone elastomer samples weighing 10±5 mg were placed in scintillation vials with 2 ml of liquid fatty acids (OL, LA and RCA) or 10 ml of fatty acid suspensions (DCA, UDA, LRA, TDA, MA, PDA, PA and STA). The vials were then kept on a shaker at 25±1°C for 48–72 h, after which the silicone elastomers were removed and rinsed with distilled deionized water several times and patted dry using Kimwipes. 5 ml of ethanol were used to extract fatty acids from the elastomers in scintillation vials kept on a medium speed shaker at 25°C for 48 h. A second extraction step using 2 ml of ethanol was conducted for 24 h to ensure complete extraction of fatty acids from the elastomers. The fatty acids in the extraction aliquots were analyzed using GC or HPLC.
HPLC and GC Assay
For OL, the same GC method described in a previous study was used (11). In the HPLC assay of RCA and DCA, the mobile phase was 65% acetonitrile: 35% of 0.1% phosphoric acid in deionized water with flow rate of 1.5 ml/min, and the detection wave length was 210 and 220 nm, respectively. E2β HPLC assay has been previously described (14). In the GC assay of UDA, MA, TDA, PDA, LRA, PA, STA and LA, the injector temperature, FID detector temperature, and column oven temperature were: 400°C, 400°C, 75°C to 300°C at a rate of 40°C/min for UDA; 350°C, 350°C, 100°C for 1 min then to 320°C at a rate of 45°C/min and held at 320°C for 4 mins for MA; 400°C, 400°C, 150°C to 320°C at a rate of 30°C/min for TDA and PDA; 350°C, 350°C, 100°C for 1 min to 300°C at a rate of 50°C/min for LRA; 400°C, 400°C, 150°C for 1 min to 320°C at a rate of 55°C/min for PA; 400°C, 400°C, 100°C for 1 min to 360°C at a rate of 60°C/min for STA; 350°C, 350°C, 80°C to 320°C at a rate of 25°C/min for LA.
RESULTS AND DISCUSSION
HEM Transport Experiments and Emax Determination
Table II shows a summary of the physicochemical properties of fatty acids used in the present study. Table III column 2 presents the permeability coefficient of CS across HEM. The result of PBS control in the table was from all experiments (23 different skin donors) in the absence of enhancers in the present study, and this result was consistent with those reported previously (6). The results of CS transport across fatty acid-treated HEM were determined under constant concentration of fatty acid in HEM. Since the transport experiments were conducted at pH 7.4, ionization and, consequently, loss of fatty acids from HEM during transport might occur. Therefore, the transport vehicle used in the experiments was PBS saturated with fatty acids. The solubility ratio of CS in fatty acid-saturated PBS to that in PBS is presented in Table III column 4. The ratios presented were used to correct for changes in the permeant thermodynamic activity in the presence of fatty acids as shown in Equation 2. The Emax of fatty acids was calculated using the ratio of the permeability coefficient across HEM in the presence of fatty acids to that of PBS control of the same skin donor (Table III column 5). The permeability coefficient of OL-treated HEM was obtained from a previous study (11), and the Emax was calculated using Equation 2 and the CS solubility ratio determined in the present study.
Table II.
A Summary of the Physicochemical Properties of the Fatty Acids: Molecular Weight (MW), Logarithm of the Octanol Water Partition Coefficient (Log Koct), Aqueous Solubility (Sw), and Melting Point. The Last Column Shows the Solubility of the Fatty Acids in Silicone Elastomer in Moles/mg of Silicone
| Fatty Acid | MW (g/mol) | Log Koct a,b | Swc (mM) | Melting Point (°C) | Silicone Uptake×107 (mole/mg)d |
|---|---|---|---|---|---|
| DCA | 172.3 | 4.02 | 0.28 | 31.9 | 23±11 |
| UDA | 186.3 | 4.51 | 0.12 | 28.6 | 6±2 |
| LRA | 200.3 | 5.00 | 0.064 | 43.2 | 0.3±0.1 |
| TDA | 214.4 | 5.49 | 0.0088 | 44.5 | NA |
| MA | 228.4 | 5.98 | 0.002 | 53.9 | 3.3±1.0 |
| PDA | 242.4 | 6.47 | 0.79×10−3 | 52.3 | 0.5±0.4 |
| PA | 256.4 | 6.96 | 0.16×10−3 | 61.8 | 3.6±0.8 |
| STA | 284.5 | 7.94 | 0.012×10−3 | 68.8 | 0.47±0.06 |
| OL | 282.5 | 7.64 | 0.04×10−3 | 13 | 0.98±0.06 |
| LA | 280.5 | 7.51 | 0.13×10−3 | −8.5 | 3.5±0.5 |
| RCA | 298.5 | 6.19 | 0.0017 | 5.5 | NA |
Calculated Log Koct obtained from EPI Suite Database, based on the chemical structure of the compound
pKa of the fatty acids ≈ 4.9, from http://aceorganic.pearsoncmg.com/epoch-plugin/public/pKa.jsp
Calculated enhancer solubility in water obtained from EPI Suite Database, based on chemical structure of the compound
Mean±SD (n=4)
Table III.
Permeability Coefficient of HEM at Emax for CS, CS Transport Lag Time, CS Solubility Ratio in Fatty Acid Saturated PBS and PBS, and Emax
| HEM treatment | Permeability coefficient of HEM for CS with direct enhancer treatment (10−7cm/s)a | Lag time (min)a | CS solubility ratio | Emaxb |
|---|---|---|---|---|
| Control | 4±2 | 47±15 | NA | NA |
| DCA | 83±36 | 49±12 | 0.91±0.02 | 14±2 |
| UDA | 46±16 | 53±25 | 1.09±0.03 | 13±3 |
| LRA | 9±3 | 46±22 | 1.00±0.02 | 6±2 |
| TDA | 15±9 | 44±4 | 1.07±0.03 | 3±2 |
| MA | 5±2 | 61±17 | 0.95±0.03 | 3±2 |
| PDA | 11±5 | 46±6 | 1.09±0.03 | 2.0±0.8 |
| PA | 3.7±0.5 | 44±6 | 1.07±0.03 | 2.4±0.7 |
| STA | 1.7±0.9 | 31±15 | 1.13±0.03 | 1.1±0.7 |
| OL | 44±19 c | 43±8 | 1.08±0.03 | 14±2 |
| LA | 31±21 | 43±17 | 0.98±0.02 | 13±4 |
| RCA | 12±6 | 25±10 | 1.10±0.03 | 3±2 |
Mean±SD (n ≥ 3) with at least three different skin donors
Emax corrected for the change in the thermodynamic activity of permeant in the presence of fatty acid, using CS solubility ratio
Data from a previous study (11)
The lag time data of CS permeation across HEM are presented in Table III column 3. The CS transport lag times were determined by extrapolating the linear regressions of the CS cumulative amount versus time plots to the abscissa. In all the transport experiments, steady-state transport was observed, and the linear regressions had r2≥0.98. The transport lag time data in the present study show that the lag times of CS permeation across HEM were relatively independent of the enhancement factor and the fatty acid treatment. This observation is similar to that of other enhancers in a previous study (14), which was investigated in a subsequent study (25).
Silicone Elastomer Enhancer Uptake
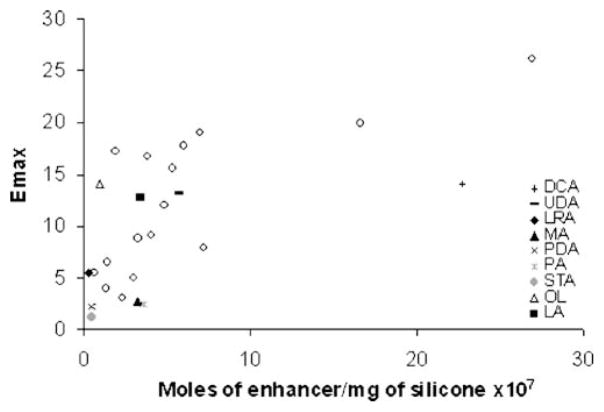
Previously, enhancer uptake in silicone elastomer has been proposed as a model for the estimation of enhancer efficiency (11). A relationship between the silicone elastomer enhancer solubility and Emax was observed, suggesting that the silicone elastomer can be used to predict enhancer efficiency and hence enhancer solubility in SC lipid domain microenvironment. The purpose of the present study was to examine this model for the evaluation of fatty acid permeation enhancement efficiency. Table II column 6 shows the fatty acid solubility in silicone elastomer. The low sensitivity of our chromatographic analysis for RCA and TDA has led to the exclusion of these two enhancers from the present study. Fig. 1 demonstrates a relationship between fatty acid Emax and silicone elastomer uptake, where previously determined data were shown in the figure by open circles (11). The results show a trend of an increase in silicone elastomer fatty acid uptake with an increase in fatty acid Emax. The linear correlation of enhancer Emax versus enhancer silicone elastomer uptake in a previous study had r2=0.57 (11), and including the fatty acid uptake data in the present study showed an increase in data scattering with r2= 0.43. This may be attributed to the affinity of fatty acids—both ionized and unionized forms—to silicone elastomer, and the inability of our analytical protocol to distinguish both species. Despite the variability, the silicone elastomer model as evident by the trend in Fig. 1 is still a relatively good model to evaluate enhancer efficiency.
Fig. 1.
Emax vs. saturated enhancer concentration in the silicone elastomer (moles/mg of silicone) determined in the silicone uptake study. The open circles represent the data of other enhancers determined previously (11).
Structure Enhancement Relationship: Emax vs. Log Koct (or Log D), Emax vs. Koct x Sw, and Emax vs. Melting Point
Previously, Emax of a number of chemical enhancers has been studied to elucidate a possible structure enhancement relationship. Emax was shown to depend on the lipophilicity of an enhancer (11). In this previous study, enhancers were found to fall into two main groups: the first (Group I) consisted of long hydrocarbon chain enhancers (number of carbons ≥ 6), and the second (Group II) consisted of enhancers with cyclic or compact hydrocarbons. Enhancers of the first group had higher Emax values when compared to enhancers of the second group at the same lipophilicity (Koct). It was hypothesized that the difference between Groups I and II was related to either the lower efficiency of Group II enhancers to fluidize SC lipids relative to Group I and/or the lower solubilities of Group II enhancers in the SC lipid domain at the same lipophilicity (11).
Fig. 2 presents a plot of enhancer Emax versus enhancer Log Koct. The open circles in the figure represent the results obtained from a previous study (11). For the fatty acids in the present study, log D (log of the distribution coefficient, log D=log (Koct/(1+10pH-pKa)) instead of log Koct was used to account for acid dissociation of the fatty acids in PBS at pH=7.4. The Emax of the fatty acids seems to follow the same trend demonstrated previously: Emax decreases with an increase in enhancer log Koct (i.e., enhancer lipophilicity). The solid fatty acids tested in the present study were shown to follow the same trend as those enhancers in Group II whereas liquid fatty acids such as OL (11) and LA follow the same trend of Group I. This indicates that the solid fatty acids have lower enhancement efficiency when compared to the liquid fatty acids. In other words, the saturated fatty acids have lower enhancement efficiency when compared to their unsaturated counterparts.
Fig. 2.
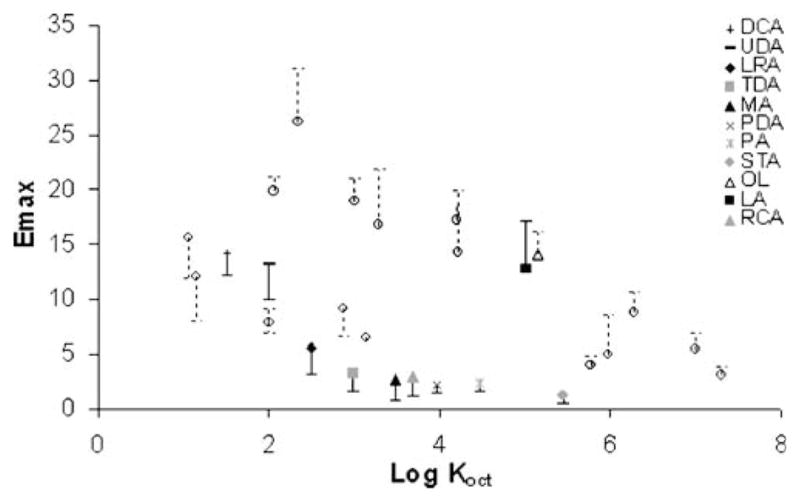
Relationship between Emax and log Koct (Log D was used for the fatty acids). The open circles represent the data of other enhancers determined previously (11).
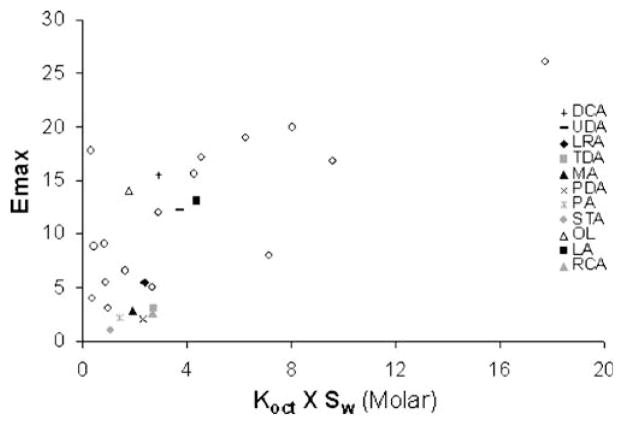
Fig. 3 shows a correlation between enhancer Emax and the calculated hypothetical enhancer solubility in n-octanol (Koct X Sw). The data from a previous study (11) are shown in the figure by open circles. As shown in Fig. 3, there is considerable data scatter with the data obtained in the previous study, possibly due to uncertainties in the Koct and Sw values of these enhancers that were both obtained from experiments as well as theoretical calculations. In the present study, the data scattering is smaller and (Koct X Sw) seems to be a reasonable model for estimating fatty acid induced permeation enhancement. The trend of an increase in fatty acid Emax with increasing fatty acid n-octanol solubility also suggests that the enhancer-targeted domain within SC lipids resembles n-octanol.
Fig. 3.
Emax vs. the product of Koct×Sw (estimated n-octanol solubility of the enhancer) where the open circles represent the data of other enhancers determined previously (11).
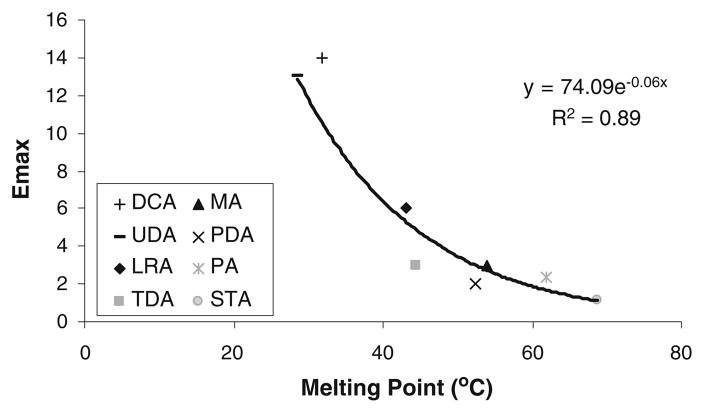
The solubilities of solid solutes are dependent on their crystallinity. Thus, unlike liquid fatty acid enhancers, the solubilities of solid fatty acids in SC lipid microenvironment also depend on their melting points. Accordingly, solid fatty acid solubility in SC lipid domain is expected to decrease with an increase in the melting point of these fatty acids, which in turn is associated with the alkyl chain length of the fatty acids; saturated fatty acids of longer alkyl chains have higher melting points. It should also be noted that solute solubility is related exponentially to solute melting point. Fig. 4 presents a plot of Emax vs. the melting points of solid fatty acids and the best fit line of Emax=a exp(-b (melting point)) where a and b are constants (r2=0.89). The observed correlation between Emax of solid fatty acids and their melting points indicates that enhancer melting point is an additional controlling parameter that influences solid enhancer efficiency. This is consistent with two observations in Fig. 2: higher magnitude of permeation enhancement induced by the liquid fatty acid enhancers compared to their saturated solid counterparts, and the decrease in Emax of the solid fatty acid enhancers with increasing Koct. The latter trend can be explained by the fact that both the Koct and melting point of the solid fatty acid enhancers are related to the alkyl chain length of the enhancers. From a theoretical viewpoint, the exponential correlation of enhancer Emax vs. enhancer melting point in Fig. 4 is consistent with enhancer solubility in the SC lipid domain being an important determinant of the effectiveness of chemical permeation enhancers.
Fig. 4.
Emax vs. the melting point of the solid fatty acids.
RCA, an unsaturated fatty acid, was tested in the present permeation enhancement study particularly due to its unique structure containing two polar moieties. The hydroxyl group on the 12th carbon of RCA could affect the effectiveness of RCA as a permeation enhancer compared to other liquid fatty acids of the same lipophilicity possibly due to complex positioning of the enhancer within the SC lipid domain. For example, RCA may be oriented in a way that would allow both polar moieties to interact with the polar domain of the SC lipids and only the hydrocarbon chain beyond the 12th carbon can be properly situated and extended into the hydrocarbon region of the lipid domain. This is supported by the lower Emax induced by RCA in comparison to other liquid fatty acids (e.g., OL and LA) in Fig. 2. It should be noted that although RCA follows the same Emax vs. n-octanol solubility relationship as the other fatty acid enhancers in Fig. 3, this could be misleading due to the use of the calculated Sw for RCA in the plot. The aqueous solubility of RCA was reported in a previous study to be 11.6 mM (26), approximately 7000 times higher than the calculated value (due to its molecular structure). On the other hand, the same study reports the aqueous solubilities of other fatty acids examined in the present study with differences between the experimental and calculated solubilities not exceeding 2–3 times. RCA would therefore be a significant outlier in Fig. 3 if its experimental solubility is used, and this might be attributed to its unique chemical structure.
Flux Enhancement with Fatty Acid Deposition from Volatile Solvent (Enhancer/Ethanol Treatment) and Duration of Flux-Enhancing Effect
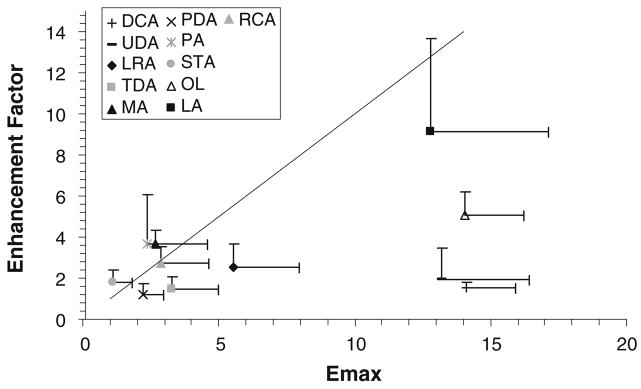
An aspect of the present study was to determine the impact of a volatile carrier solvent system (ethanol) on fatty acid deposition and evaluate fatty acid-induced transdermal permeation enhancement from this carrier system. Table IV column 3 shows the transport lag times of CS across fatty acid/ethanol-treated HEM. Table IV column 2 summarizes the permeability coefficients of CS across HEM treated with fatty acids in the ethanol solvent system. Previously, it has been suggested that enhancer deposition from a volatile solvent introduces the enhancer in its pure form on HEM that will then partition into SC where the thermodynamic activity of the enhancer approaches that of the enhancer in its pure state, and the permeation enhancement induced by the enhancer would approach its Emax (14). Fig. 5 presents a relationship between the permeation enhancement factor with fatty acid/ethanol treatment and fatty acid Emax. There is no statistically significant difference between the permeation enhancement factors and Emax of the fatty acids (p>0.05) with the exception of DCA, UDA, LRA, and OL. The deviation of DCA, UDA, and to a lesser extent LRA from the enhancement factor of fatty acid/ethanol treatment vs. Emax correlation in the figure is believed to be related to enhancer depletion from the SC lipids and will be discussed in the next paragraph. To understand the deviation of OL, a separate study found that the uptake of OL into the SC lipid domain after OL/ethanol treatment was below the detection limit in the GC assay (<0.001 v/v). This suggests ineffective deposition of OL on the surface of HEM and consequently lower OL uptake into SC when compared to pure OL treatment.
Table IV.
Permeability Coefficient of HEM, Lag Time, and Transport Enhancement Factor Across HEM Induced by Fatty Acid Deposition from Ethanol Solution
| HEM treatment | Permeability coefficient of HEM for CS with enhancer/ethanol formulation (10−7cm/s)a | Lag time (min)a | Enhancement factorb |
|---|---|---|---|
| Control/Ethanol | 4±2 | 56±4 | NA |
| DCA | 6±2 | NAc | 1.5±0.3 |
| UDA | 10±3 | NAc | 2±2 |
| LRA | 7±4 | 31±12 | 2.6±0.9 |
| TDA | 6±3 | 36±16 | 1.4±0.6 |
| MA | 6.3±0.5 | 46±15 | 3.7±0.7 |
| PDA | 5±1 | 29±19 | 1.2±0.6 |
| PA | 7±3 | 43±17 | 4±2 |
| STA | 3±1 | 53±5 | 1.7±0.6 |
| OL | 14±10 | 49±1 | 5±1 |
| LA | 13±7 | 44±3 | 9±5 |
| RCA | 10±2 | 23±7 | 2.7±0.9 |
Mean±SD (n ≥ 3) with at least three different skin donors
Enhancement factor determined with HEM after fatty acid/ethanol treatment (ethanol is the volatile carrier)
The lag time is not reported due to the large variability attributed to enhancer depletion in the transport experiment
Fig. 5.
Enhancement factor determined after fatty acid enhancer deposition on HEM with the volatile solvent versus Emax of the fatty acid. The line shows the slope of unity.
DCA and UDA have log D of 1.5 and 2, respectively. The relatively low log D values of these fatty acid enhancers would suggest enhancer depletion from HEM in the permeation experiment (11) after fatty acid/ethanol treatment, thus resulting in the lower enhancement factors of DCA and UDA than their Emax. The result also suggests the inability of the relatively low lipophilic fatty acids to maintain their enhancement effect after the enhancers were deposited on HEM. This explanation is consistent with the trend of the enhancement factor for DCA, UDA, and LRA (1.5, 2, and 2.6, respectively) showing a higher degree of HEM barrier recovery with a decrease in enhancer log D, i.e., a higher loss of enhancer from SC as enhancer lipophilicity decreases. From a mechanistic viewpoint, the reversibility of HEM barrier after treatment with the low lipophilic fatty acids suggests no SC lipid extraction that would significantly affect CS permeation across the SC lipoidal pathway.
Many chemical penetration enhancers are solids such as the long chain fatty acids examined in the present study (27). The mechanism of solid chemical enhancers to exert their permeation enhancement effect in topical products when the enhancers are deposited as solids from a volatile carrier solvent(s) on HEM is not fully understood. The present results provide insights into the effects of the volatile solvent delivery system upon permeation enhancement of the solid chemical enhancers. It is believed that the uptake of an enhancer in its solid state into HEM would require the dissolution of the solid enhancer deposited on the HEM surface in the SC lipids, in the water permeating through HEM from the wetted support, and/or in the water from the humidity in the environment. The permeation enhancement data in the present volatile solvent study suggest the deposition of the solid fatty acid enhancer on HEM surface would eventually result in enhancer permeation and partitioning into SC, providing enhancement effects similar to Emax (Fig. 5).
SC E2β Uptake Enhancement with Fatty Acid Deposition from Volatile Solvent
Previously, the impact of enhancers on permeant partitioning has been determined, and it was proposed that enhancer-induced permeation enhancement is attributed to the enhancement of permeant partitioning (14). The present study investigated if the mechanism of action of fatty acids on skin permeation enhancement would be similar to that previously determined. Table V summarizes the amount of E2β uptake in SC (n-hexane and delipidized) after fatty acid/ethanol treatment of SC. E2β uptake into the lipid domain of fatty acid/ethanol-treated SC was calculated by subtracting the amount uptake in the delipidized SC from that in n-hexane-treated SC [(Table V column 2)-(Table V column 4)]. OL and PDA-treated SC samples show no significant difference between E2β uptake in n-hexane-treated and delipidized SC samples, and therefore E2β uptake into the lipid domain of SC treated with these enhancers has been excluded from the analyses. It is speculated that OL and PDA may interact with the delipidized compartments in SC and increase the E2β uptake into the delipidized SC domain.
Table V.
Estradiol Uptake in N-Hexane-Treated and Delipidized Human Stratum Corneum in the Enhancer/Ethanol Treatment Study
| Amount of E2β Uptake into n-Hexane-Treated Human SCa |
Amount of E2β Uptake into Delipidized Human SCa |
||
|---|---|---|---|
| SC Treatment | Ecorrected,ib (micromoles/mg Dry n-Hexane Treated Human SC) | Ecorrected,ib (micromoles/mg Dry Delipidized Human SC) | Ecorrected,ic (micromoles/mg Dry Delipidized Human SC) |
| Ethanol (EtOH)d | 0.03±0.02 | 0.012±0.005 | 0.011±0.005 |
| DCA/EtOH | 0.06±0.04 | 0.02±0.02 | 0.02±0.01 |
| UDA/EtOH | 0.06±0.05 | 0.01±0.01 | 0.01±0.01 |
| LRA/EtOH | 0.11±0.06 | 0.02±0.02 | 0.016±0.016 |
| TDA/EtOH | 0.05±0.01 | 0.02±0.02 | 0.01±0.01 |
| MA/EtOH | 0.04±0.02 | 0.0062±0.0006 | 0.0052±0.0005 |
| PDA/EtOH | 0.03±0.02 | 0.06±0.07 | 0.05±0.06 |
| PA/EtOH | 0.08±0.02 | 0.0041±0.0009 | 0.0035±0.0007 |
| STA/EtOH | 0.023±0.007 | 0.013±0.005 | 0.011±0.004 |
| OL/EtOH | 0.03±0.01 | 0.05±0.01 | 0.04±0.01 |
| LA/EtOH | 0.3±0.1 | 0.024±0.007 | 0.021±0.005 |
Mean±SD (n ≥ 4)
Corrected for the uptake into the aqueous compartment (Equation 4)
Normalized by the weight of n-hexane-treated SC. Hence, the uptake data were multiplied by the weight percent of the delipidized component of SC (~ 84%)
Data presented in a previous study (11)
Fig. 6 shows a correlation between Emax and the ratio of E2β uptake into SC lipid domain of enhancer-treated SC to control SC (uptake enhancement ratio). The linear regression with a slope of 1.0 (r2 =0.88) suggests that fatty acid-induced permeation enhancement is mainly attributed to permeant partitioning enhancement, similar to that observed previously with enhancers of other chemical classes (14).
Fig. 6.
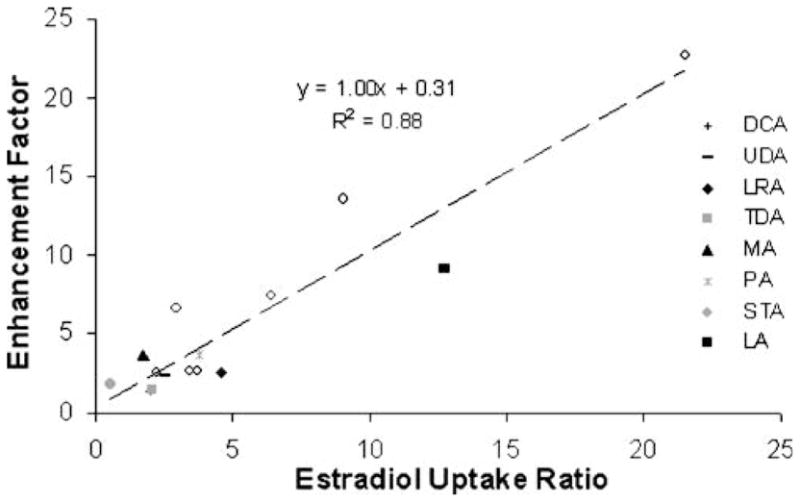
Relationship between CS permeation enhancement factor and the ratio of E2β uptake in the intercellular lipid domain of enhancer/ethanol-treated SC to ethanol-treated control SC. The open circles represent the data of other enhancers determined previously (14).
Fatty Acids as Transdermal Permeation Enhancers
The present study evaluated the permeation enhancement efficiency of a number of fatty acids commonly used in topically applied formulations. Due to the lipophilic nature of these fatty acids, most studies elucidating their percutaneous permeation enhancement were carried out in the presence of cosolvents or solubilizing agents. The purpose of the present study was to evaluate the efficiency of fatty acids as permeation enhancers applied to the skin as pure compounds without cosolvents. This situation of enhancers without cosolvents can be found in the application of transdermal and topical gels and volatile transdermal sprays after the solvent evaporates. The first main finding in the present study is that the liquid fatty acids (e.g., OL and LA) have relatively higher efficiency compared to the solid fatty acids (e.g., STA and TDA) as permeation enhancers. The solid fatty acids of relatively high lipophilicity or high melting points have low permeation enhancement effect. As a result, these solid fatty acids would not be as effective as the liquid fatty acids as permeation enhancers in topically applied formulations under the conditions investigated in the present study. Second, the solubilities of enhancers in the SC lipid domain remain an important factor for effective transdermal permeation enhancement for the fatty acid enhancers, and there exists a relationship between permeation enhancement of the enhancers and enhancer solubilities in silicone elastomer. Third, enhancer lipophilicity could be an indicator of the duration of enhancement induced by the enhancer. In other words, as enhancer lipophilicity decreases, the extent of enhancer depletion from HEM increases, and consequently the permeation enhancement effect cannot be maintained. In addition, these fatty acids of lower lipophilicity generally have higher aqueous solubility, and this would result in a pH change in the formulation and possibly skin irritation and structural denaturation. For the solid fatty acids with relatively high lipophilicity, they could maintain a longer duration of enhancement but would result in lower permeation enhancement.
CONCLUSION
The ability to elucidate a structure enhancement relationship of chemical permeation enhancers would facilitate the selection of enhancers for topical and transdermal pharmaceutical products. Particularly, fatty acids are widely used in pharmaceutical and cosmetic products and in many formulations as permeation enhancers. The enhancement effects of fatty acids have been determined in many studies, yet the sole impact of fatty acids in the absence of cosolvents and solubilizing agents has never been systematically studied. In a recent study, Emax was proposed as a model to study enhancer efficiency in topical and transdermal products that deposit an infinite dose of the enhancer on skin (14). Examples of these products that consist of volatile solvent systems are transdermal and topical hydro-alcoholic gel, spray, and aerosol. It has been hypothesized that the deposition of an enhancer on skin from these formulations would result in the enhancer saturating the SC lipids and subsequent permeation enhancement under a condition similar to that of Emax. In the present study, a general correlation was found between Emax and permeation enhancement induced by fatty acid deposition from a volatile solvent. Furthermore, the present study supports the possibility of using solid fatty acids as permeation enhancers in formulations such as those discussed earlier, though the kinetics of solid dissolution may in some cases be a limiting factor. In the SC uptake study, the mechanism of fatty acids as permeation enhancers was found to be mainly through the enhancement of permeant partitioning into the SC intercellular lipid domain. Furthermore, the findings in the present study are consistent with those in a previous study with enhancers of different chemical classes (11). Particularly, the Emax of the studied fatty acids seems to follow the previously determined Emax vs. enhancer lipophilicity relationship. The Emax vs. Koct X Sw relationship also suggests the absence of specific interactions between the enhancers and SC lipid domain as a mechanism of action. In addition, the relationship between fatty acid Emax vs. fatty acid silicone elastomer uptake supports the use of silicone elastomer as a model for the evaluation of fatty acid/enhancer efficiency.
Acknowledgments
This research was supported in part by NIH Grant GM 063559. The authors thank Dr. Jinsong Hao for her help in the laboratory.
References
- 1.Al-Qallaf B, Das DB, Mori D, Cui Z. Modelling transdermal delivery of high molecular weight drugs from microneedle systems. Philosophical Transactions of the Royal Society a-Mathematical Physical and Engineering Sciences. 2007;365:2951–67. doi: 10.1098/rsta.2007.0003. [DOI] [PubMed] [Google Scholar]
- 2.Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc Natl Acad Sci U S A. 2005;102:4688–93. doi: 10.1073/pnas.0501176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansel HC, Allen LV, Popovich NG. Pharmaceutical dosage forms and drug delivery systems. Philadelphia: Lippincott-Williams & Wilkins; 1999. [Google Scholar]
- 4.El-Gibaly I, Mohamed FA, Shehata M. Effect of some penetration enhancers on release of clotrimazole from different gel formulations and histological changes of rabbit skin. Pharmazeutische Industrie. 1998;60:1088–95. [Google Scholar]
- 5.Finnin BC, Morgan TM. Transdermal penetration enhancers: applications, limitations, and potential. J Pharm Sci. 1999;88:955–8. doi: 10.1021/js990154g. [DOI] [PubMed] [Google Scholar]
- 6.Chantasart D, Sa-Nguandeekul P, Prakongpan S, Li SK, Higuchi WI. Comparison of the effects of chemical permeation enhancers on the lipoidal pathways of human epidermal membrane and hairless mouse skin and the mechanism of enhancer action. J Pharm Sci. 2007;96:2310–26. doi: 10.1002/jps.20865. [DOI] [PubMed] [Google Scholar]
- 7.Ogiso T, Paku T, Iwaki M, Tanino T. Percutaneous penetration of fluorescein isothiocyanate-dextrans and the mechanism for enhancement effect of enhancers on the intercellular penetration. Biol Pharm Bull. 1995;18:1566–71. doi: 10.1248/bpb.18.1566. [DOI] [PubMed] [Google Scholar]
- 8.Goates CY, Knutson K. Enhanced permeation and stratum corneum structural alterations in the presence of dithiothreitol. Biochim Biophys Acta. 1993;1153:289–98. doi: 10.1016/0005-2736(93)90418-y. [DOI] [PubMed] [Google Scholar]
- 9.Niazy EM. Influence of oleic-acid and other permeation promoters on transdermal delivery of dihydroergotamine through rabbit skin. Int J Pharm. 1991;67:97–100. [Google Scholar]
- 10.Chantasart D, Li SK, He N, Warner KS, Prakongpan S, Higuchi WI. Mechanistic studies of branched-chain alkanols as skin permeation enhancers. J Pharm Sci. 2004;93:762–79. doi: 10.1002/jps.10550. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim SA, Li SK. Effects of chemical enhancers on human epidermal membrane: Structure-enhancement relationship based on maximum enhancement Emax. J Pharm Sci. 2009;98:926–44. doi: 10.1002/jps.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karande P, Jain A, Mitragotri S. Insights into synergistic interactions in binary mixtures of chemical permeation enhancers for transdermal drug delivery. J Control Release. 2006;115:85–93. doi: 10.1016/j.jconrel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Morgan TM, Parr RA, Reed BL, Finnin BC. Enhanced transdermal delivery of sex hormones in swine with a novel topical aerosol. J Pharm Sci. 1998;87:1219–25. doi: 10.1021/js980026c. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim SA, Li SK. Effects of solvent deposited enhancers on transdermal permeation and their relationship with Emax. J Control Release. 2009;136:117–24. doi: 10.1016/j.jconrel.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper ER. Increased skin permeability for lipophilic molecules. J Pharm Sci. 1984;73:1153–6. doi: 10.1002/jps.2600730831. [DOI] [PubMed] [Google Scholar]
- 16.Barry BW, Bennett SL. Effect of penetration enhancers on the permeation of mannitol, hydrocortisone and progesterone through human skin. J Pharm Pharmacol. 1987;39:535–46. doi: 10.1111/j.2042-7158.1987.tb03173.x. [DOI] [PubMed] [Google Scholar]
- 17.Kandimalla K, Kanikkannan N, Andega S, Singh M. Effect of fatty acids on the permeation of melatonin across rat and pig skin in-vitro and on the transepidermal water loss in rats in-vivo. J Pharm Pharmacol. 1999;51:783–90. doi: 10.1211/0022357991773140. [DOI] [PubMed] [Google Scholar]
- 18.Aungst BJ. Structure/effect studies of fatty acid isomers as skin penetration enhancers and skin irritants. Pharm Res. 1989;6:244–7. doi: 10.1023/a:1015921702258. [DOI] [PubMed] [Google Scholar]
- 19.Aungst BJ, Rogers NJ, Shefter E. Enhancement of naloxone penetration through human skin in vitro using fatty-acids, fatty alcohols, surfactants. sulfoxides and amides. Int J Pharm. 1986;33:225–34. [Google Scholar]
- 20.Ongpipattanakul B, Burnette RR, Potts RO, Francoeur ML. Evidence that oleic acid exists in a separate phase within stratum corneum lipids. Pharm Res. 1991;8:350–4. doi: 10.1023/a:1015845632280. [DOI] [PubMed] [Google Scholar]
- 21.Nanayakkara GR, Bartlett A, Forbes B, Marriott C, Whitfield PJ, Brown MB. The effect of unsaturated fatty acids in benzyl alcohol on the percutaneous permeation of three model penetrants. Int J Pharm. 2005;301:129–39. doi: 10.1016/j.ijpharm.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Yokomizo Y, Sagitani H. The effects of phospholipids on the percutaneous penetration of indomethacin through the dorsal skin of guinea pig in vitro. 2. The effects of the hydrophobic group in phospholipids and a comparison with general enhancers. J Control Release. 1996;42:37–46. [Google Scholar]
- 23.Kasting GB, Bowman LA. Electrical analysis of fresh, excised human skin: a comparison with frozen skin. Pharm Res. 1990;7:1141–6. doi: 10.1023/a:1015928225089. [DOI] [PubMed] [Google Scholar]
- 24.Warner KS, Li SK, He N, Suhonen TM, Chantasart D, Bolikal D, Higuchi WI. Structure-activity relationship for chemical skin permeation enhancers: probing the chemical microenvironment of the site of action. J Pharm Sci. 2003;92:1305–22. doi: 10.1002/jps.10367. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim SA, Li SK. Chemical enhancer solubility in human stratum corneum lipids and enhancer mechanism of action on stratum corneum lipid domain. Int J Pharm. 2010;383:89–98. doi: 10.1016/j.ijpharm.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yalkowsky SH, Dannenfelser RM. Aquasol Database of Aqueous Solubility. Version 5. 1992. [DOI] [PubMed] [Google Scholar]
- 27.Nair VB, Panchagnula R. Effect of iontophoresis and fatty acids on permeation of arginine vasopressin through rat skin. Pharmacol Res. 2003;47:563–9. doi: 10.1016/s1043-6618(03)00016-1. [DOI] [PubMed] [Google Scholar]