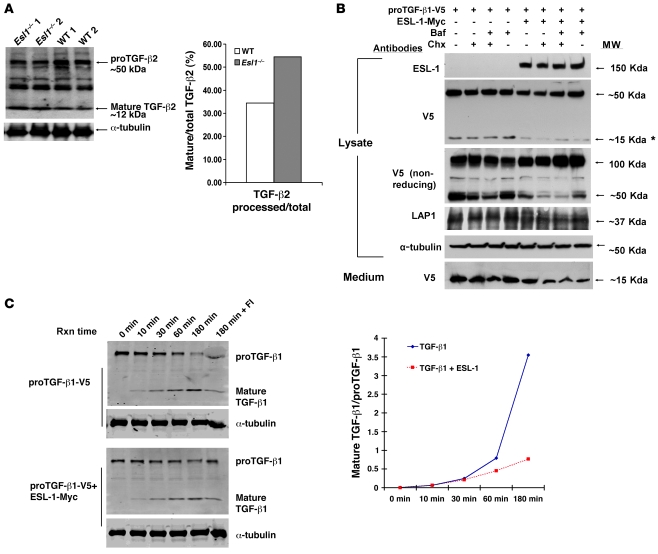
Figure 7. ESL-1 inhibits TGF-β proteolytic processing.
(A) ESL-1 inhibits the intracellular cleavage and maturation of proTGF-β2 in vivo as shown by the relative abundance of mature TGF-β2 and proTGF-β2 on Western blot analysis of Esl1–/– versus WT cartilage lysates. α-Tubulin is used as a loading control. The ratio of mature TGF-β2 to total TGF-β2 is shown at right. (B) ESL-1 inhibits the intracellular cleavage of proTGF-β1 in vitro. The scheme of transfections and chemical treatments of COS7 cells is shown at the top. Primary antibodies and molecular weights are denoted. Both proTGF-β1-V5 (50 kDa) and the mature TGF-β1-V5 (15 kDa) can be detected by V5 antibody. Note that the amount of 15-kDa TGF-β1 (*) was significantly reduced in the presence of ESL-1. Under nonreducing condition, the presence of ESL-1 remarkably increased proTGF-β1 dimer (100 kDa). LAP1 (37 kDa) was decreased in the presence of ESL-1. α-Tubulin, as an internal control, was similar in all samples. Expression of ESL-1 reduced the secretion of mature TGF-β1 ligand found in medium. Baf, bafilomycin; Chx, cycloheximide. (C) ESL-1 inhibits furin processing of proTGF-β1 by in vitro furin assay. Western blot analysis of the furin reaction samples with V5 antibody. Reaction time (Rxn time), plasmids for transfections, and identity of the bands are noted. Furin inhibitor II (hexa-d-arginine) was added in the last sample (180 min + FI) to confirm the specificity of the furin reaction. The ratios of cleaved to uncleaved TGF-β1 at all time points is shown. The proportion of mature TGF-β1 ligand is greatly increased after 30 minutes incubation in the absence of ESL-1.