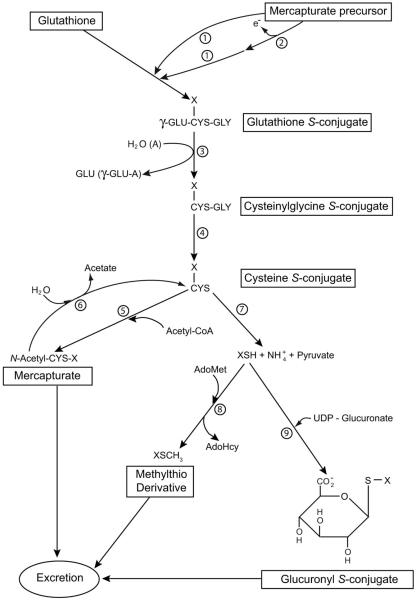
Fig. 1.
The mercapturate pathway and associated side reactions. If the mercapturate precursor contains an electrophilic center it may react directly with GSH (reaction 1). Alternatively, the precursor may be converted to a compound with an electrophilic center (reaction 2) prior to reaction with GSH. Reactions 1 through 5 represent the mercapturate pathway. Reactions 7, 8 and 9 are alternative reactions for elimination of the cysteine S-conjugate. Reactions 7 plus 8 denote the thiomethyl shunt. The thiomethyl compound (XSCH3) may be excreted unchanged or further oxidized to sulfoxide, sulfone or CO2 and sulfate, which are excreted. For some cysteine S-conjugates metabolism may also involve conversion to the α-keto acid, α-hydroxy acid, oxidatively decarboxylated product or sulfoxide (not shown). Enzymes: 1) glutathione S-transferases, 2) oxidases that generate an electrophilic center for attack by GSH (in some cases oxidation may be non-enzymatic); 3) γ-glutamyltransferase (GGT); 4) dipeptidases; 6) aminoacylases; 7) cysteine S-conjugate β-lyases; 8) thiomethyltransferase; 9) UDP-glucuronosyltransferases. In vivo the hydrolysis reaction of GGT predominates over the formation of γ-glutamyl amino acids. Abbreviations: AdoHcy, S-adenosyl-L-homocysteine; AdoMet, S-adenosyl-L-methionine; A, amino acid, dipeptide or GSH acceptor for the γ-glutamyltransferase reaction; γ-GLU-A, γ-glutamyl amino acid (or γ-glutamyldipeptide; γ-glutamylglutathione). Many potentially toxic xenobiotics and a few endogenous compounds are metabolized through the mercapturate pathway. Modified in part from Silbernagl and Heuner (1993) and Cooper and Pinto (2008). Reproduced from Cooper and Hanigan (2010) with permission.