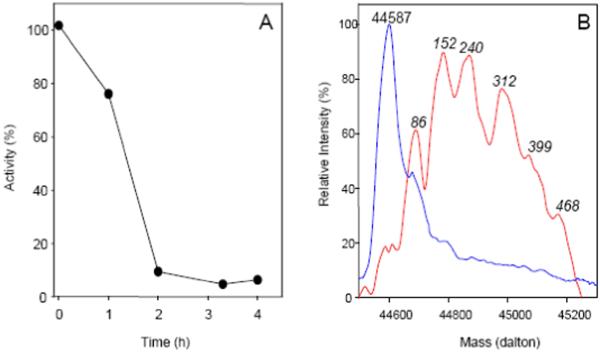
Fig 2.
Syncatalytic inactivation of mAAT by TFEC. A. Inactivation of mAAT (0.1 mg/ml) in the presence of 10 mM sodium phosphate buffer (pH 7.5), 10 mM α-ketoglutarate and 10 mM TFEC; 10°C. Enzyme activities (●) are expressed as percent relative to the activity of a sample of native enzyme maintained under identical conditions, but in the absence of TFEC. B. Mass spectra of mAAT. Mass spectra were obtained before (blue line) and after 4 h of reaction with TFEC (red line). The protein aliquots were desalted in a C8 reverse phase column and injected into the mass spectrometer. Mass spectra were deconvoluted using the algorithm included in the Xcalibur software. For the covalently modified protein the masses are given as increment in mass relative to the mass of the mAAT polypeptide (44,587 daltons).