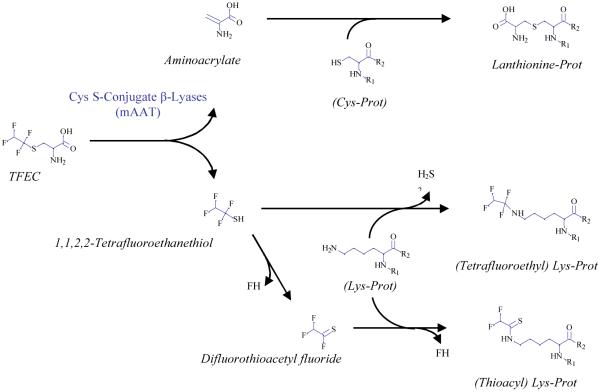
Fig. 4.
Reaction pathways of TFEC with mAAT. Aminoacrylate reacts with sulfhydryl groups of cysteine (Cys) residues resulting in the formation of lanthionine residues. The other product, tetrafluoroethanethiol, can react directly or after decomposition to difluorothioacetyl fluoride, with the ε-amino group of K residues. In the first case, loss of H2S results in the formation of a tetrafluoroethyl derivative. In the second case, loss of FH results in the formation of a thioacyl derivative.