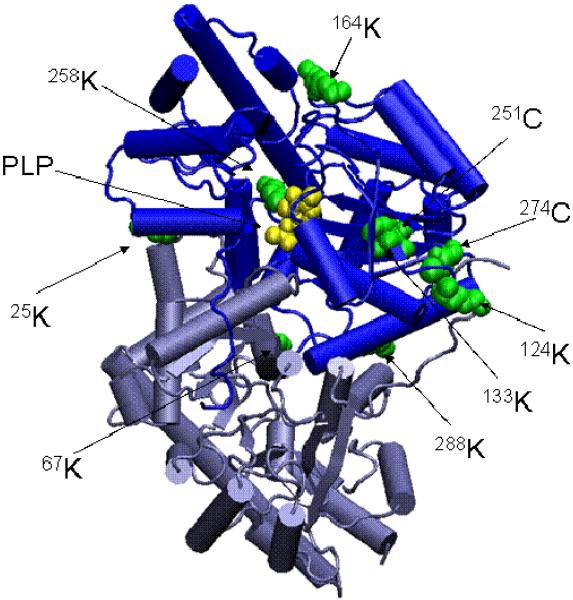
Fig. 5.
Three dimensional structure of mAAT indicating residues modified by covalent attachment of TFEC fragments. The relative positions of the modified residues are shown in green on the native structure of mAAT. The PLP coenzyme is shown in yellow forming a Schiff base with the Lys 258. The diagram was generated using the program VMD (Theoretical Biophysics Group, University of Illinois at Urbana-Champaign) and the X-ray structures of mAAT (pyridoxal form, Protein Data Bank entry code 1TAT) from chicken heart mitochondria.