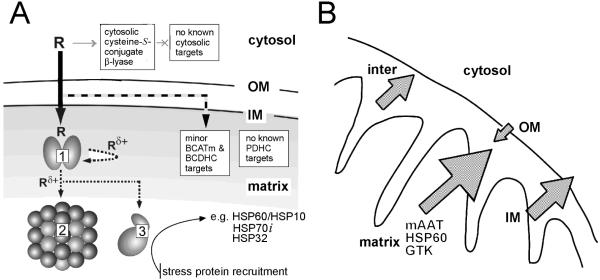
Fig. 6.
A. Mitochondrial toxicant channeling in vivo. TFEC (R) transport into the mitochondria results in conversion to the thioacylating agent (Rδ+) by β-lyases [e.g. mAAT (1), shown here as a homodimer]. Co-immunoprecipitation and biochemical studies confirm the close juxtaposition of other DFTAL target proteins – especially those of energy metabolism [KGDHC (2) and mitACON (3)]. Considerable evidence suggests that BCATm can form a metabolon with the branched-chain α-keto acid dehydrogenase complex (BCDHC) (Islam et al., 2010 and references quoted therein). Since BCATm can catalyze a β-lyase reaction with TFEC (Cooper et al., 2003) it is probable that subunits of the BCDHC are also inactivated by toxicant channeling. Other complexes not known to be associated with any aminotransferase/cysteine S-conjugate β-lyase activities [e.g. PDC] are not modified or inactivated by thioacylation. The curved arrow represents “self-thioacylation” of mAAT (syncatalytic inactivation). Positions of outer (OM) and inner (IM) mitochondrial membranes are indicated. Adapted from Cooper et al. (2002a). B. TFEC-induced mitochondrial pathophysiology. Submitochondrial fractionation studies confirm a unified movement of matrix, IM and inter-membrane space DFTAL-labeled and unmodified proteins to the periphery of rat renal mitochondria isolated from kidney tissue treated with TFEC. Such movements are consistent with a permeability transition and Δψi collapse as assessed by morphological changes, flow cytometry and biochemical inhibitor studies (see the text for details). Note that DFTAL covalent modifications of mAAT, KGDHC components, mitACON, HSP70, and HSP60 were detected in kidney mitochondria of rats exposed in vivo to TFEC by immunochemical methods. This finding does not, however, preclude the possibility of additional covalent modifications occurring in vivo as has been demonstrated for mAAT in vitro as shown in Fig. 4. Adapted from Bruschi et al. (1993).