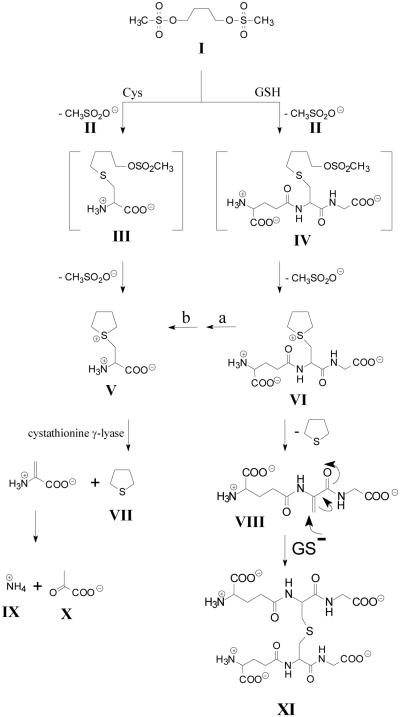
Fig. 8.
Proposed mechanism for the metabolic conversion of busulfan to tetrahydrothiophene (THT) and to a glutathione adduct containing a lanthionine bridge. Facile elimination of a methanesulfonyl (II) group from busulfan (I) occurs by nucleophilic attack of cysteine (Cys) or glutathione (GSH), resulting in the formation of thioether conjugates [cysteine S-conjugate (III) and glutathione S-conjugate (IV), respectively]. Conversion of I to IV may occur non-enzymatically or may be accelerated by various GSTs. Structures III and IV spontaneously undergo elimination/ring closure to generate sulfonium conjugates [cysteine S-conjugate (V) and glutathione S-conjugate (VI), respectively]. Structure VI is converted to V by the action of γ-glutamyltransferase (a) and dipeptidases (b), both of which possess broad specificities. V undergoes a facile β-elimination reaction to yield THT (VII), ammonium (IX) and pyruvate (X), which may be catalyzed by OH-, PLP or several PLP-containing enzymes. THT (VII) may also be generated from γ-E-THT-AG (VI) by a non-enzymatic β-elimination reaction that results in the formation of γ-glutamyldehydroalanylglycine (EDAG) (VIII). Note that the sulfur in THT is not derived from busulfan, but rather from the sulfur of GSH. VIII contains an activated vinyl group and spontaneously undergoes a Michael reaction with GSH to form structure XI that contains a lanthionine bridge. Modified from Cooper et al. (2008b) and Younis et al. (2008)