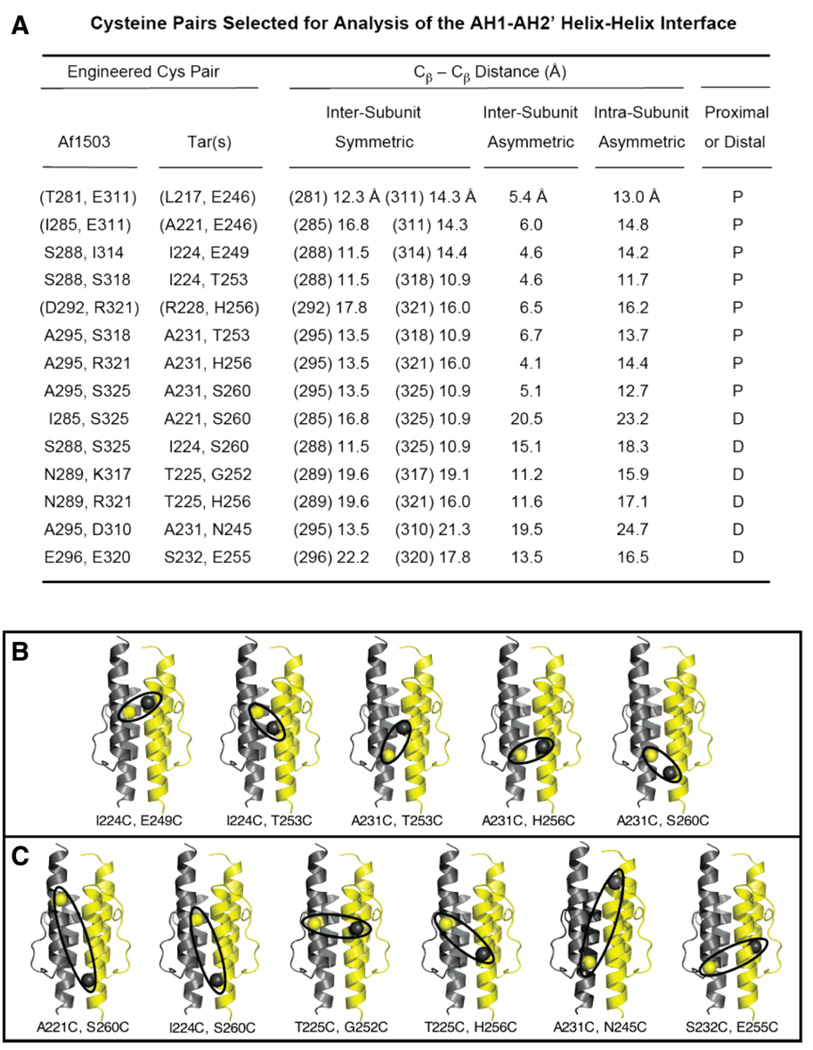
Figure 2.
Cys pairs selected for analysis of the HAMP AH1–AH2′ helical interface. (A) Summary of pairs of positions selected in the archeal HAMP structure (6), and the corresponding pairs in the S. typhimurium aspartate receptor. Also shown are the four β-carbon–β-carbon distances measured for each pair in the homodimeric, archeal HAMP structure. Each proximal pair (P) possesses an asymmetric, intersubunit distance of < 7 Å, while the other three distances simultaneously exceed 10 Å. In each distal pair (D), all four distances exceed 10 Å. Parentheses indicate low-yield di-Cys mutants not suitable for further analysis (see the text). (B and C) Locations of the proximal (B) and distal (C) Cys pairs employed for disulfide mapping. Ovals highlight the asymmetric, intersubunit separation for each pair.