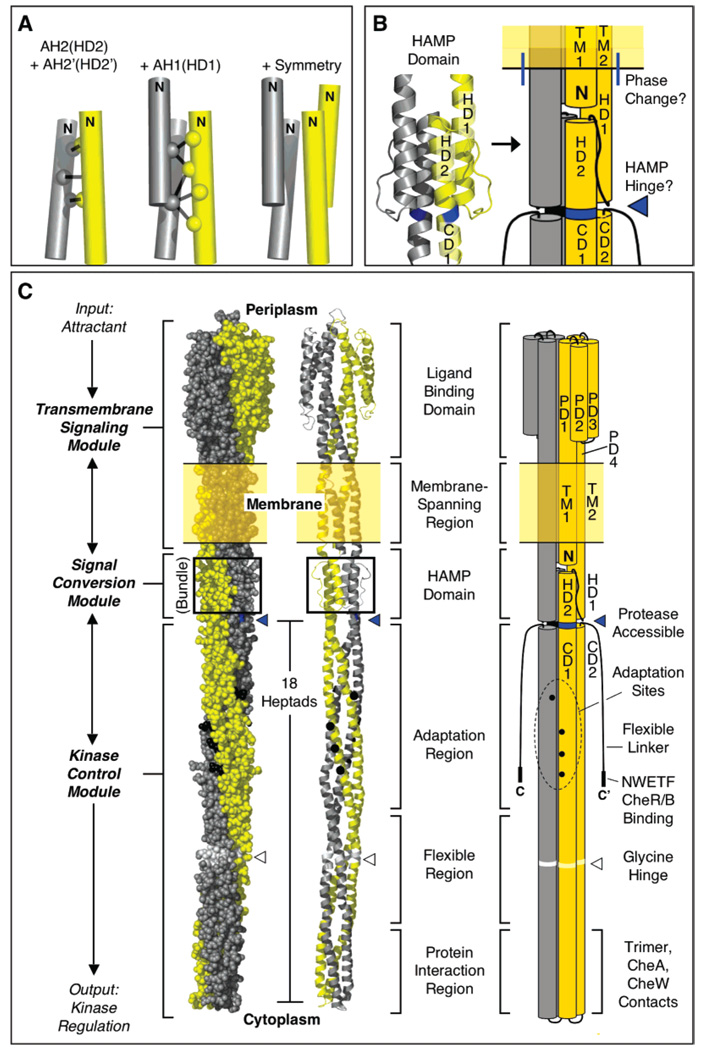
Figure 5.
Model for HAMP structure in the full-length aspartate receptor. (A) The model is built using disulfide mapping constraints (4) to define the geometry of the AH2–AH2′ helix pair, then building in the AH1–AH2′ interaction based on additional disulfide mapping constraints (Figure 3), and finally using the known C2 symmetry of the homodimer to complete the parallel, four-helix bundle (see the text). (B and C) Schematic model incorporating HAMP into the full-length receptor, illustrating a new helix nomenclature. For clarity, the model is shown in space-filling, ribbon, and cylinder formats which together portray the overall shape, subunit supercoiling, and helical structure of the homodimer. Highlighted is (i) the junction between the end of helix TM2 and the beginning of HAMP helix HD1, where there is a helical phase change (see the text), and (ii) the major proteolysis site, Arg259. The protease accessibility of the latter site suggests that position 259 lies at or near C-terminus of the distinct HAMP structural domain. The model of the full-length homodimer illustrates the three distinct modules (transmembrane signaling, signal conversion, and kinase control) of the receptor, as well as the three functional regions (adaptation, flexible, and protein interaction) of the latter module (3, 27).