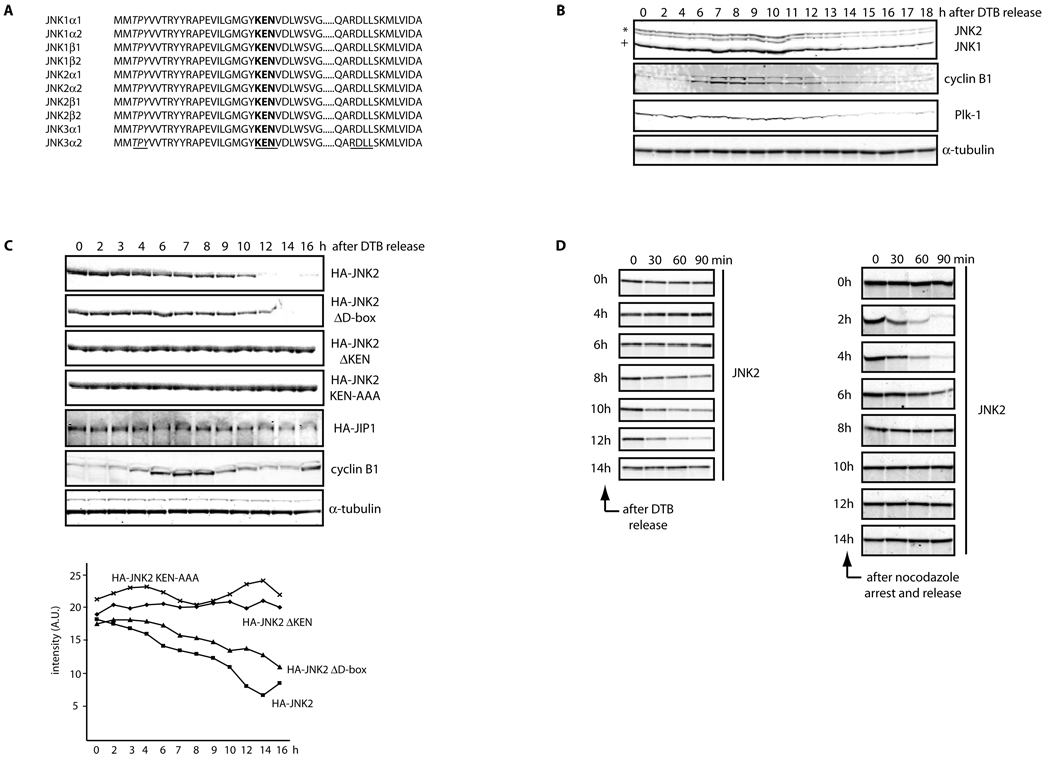
Figure 1. JNK is degraded in vivo and in vitro in a cell cycle- and KEN box-dependent manner.
(A) Multi-alignment of a selected protein sequence from the ten members of the human JNK family. Highlighted in italics and underlined is the activation loop (amino acids 183–185, in JNK2α2); in bold and underlined is the KEN box (amino acids 203–205, in JNK2α2) and underlined a putative Destruction-box (D-box) (amino acids 295–298, in JNK2α2). (B) Extracts prepared from HeLa cells were synchronized by a double-thymidine block (DTB) and analyzed over a period of 18 h by immunoblotting using the indicated antibodies. JNK2 displays as a 54kDa band (*) while JNK1 displays as a 46kDa band (+). FACS analysis confirming the synchronization is shown in Figure S1A. (C) Synchronized HeLa cells overexpressing either HA-tagged JNK2 (wild-type or mutants) or HA-tagged JIP1 (JNK Interacting Protein 1) or control-transfected cells were analyzed by immunoblotting with antibodies against HA-tag, cyclin B1, or tubulin. Quantification of JNK2 (wild-type or mutants) levels for each synchronization is shown in the graph. (D) JNK2 in vitro degradation assays in concentrated extracts prepared from HeLa cells released after being synchronized by either DTB (left panels) or nocodazole arrest (right panels). FACS data is included in Figure S1G. (E) JNK2 in vitro degradation assays in Xenopus laevis egg extracts. Uncropped images for key results of this figure are shown in Figure S7.