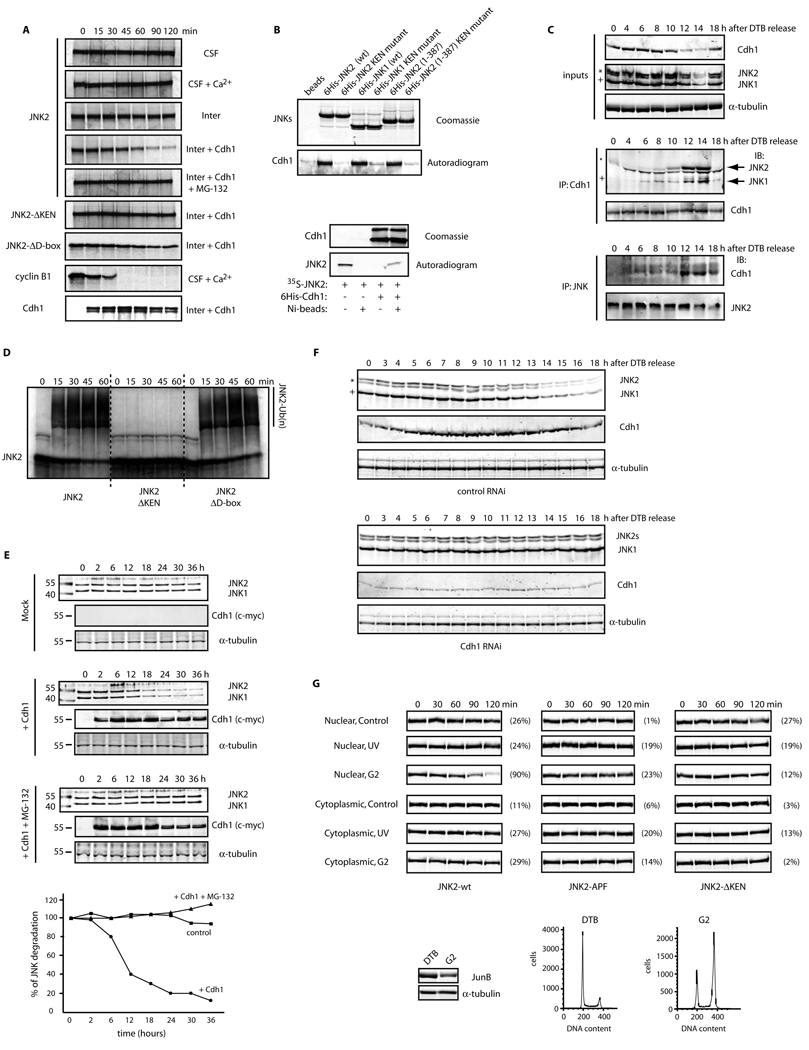
Figure 2. JNK levels are directly regulated by APC/CCdh1-mediated protein degradation during the cell cycle.
(A) Top panels: in vitro binding assay using recombinant 6×His-tagged JNKs and Cdh1/fzr translated in reticulocyte lysates and radiolabeled with 35S-methionine. Bottom panels: in vitro binding assay using recombinant 6×His-tagged Cdh1 and JNK2 translated in reticulocyte lysates and radiolabeled. Autoradiograms and Coomassie-stained gels are shown. (B) In vivo binding between endogenous JNKs and endogenous Cdh1 immunoprecipitated from synchronized HeLa cells released from a double-thymidine block (DTB). JNK2 displays as a 54kDa band (*) while JNK1 displays as a 46kDa band (+). (C) In vitro ubiquitination assay using JNK2 (wild-type or mutants) and immunoprecipitated APC/C complex from exponentially growing HeLa cells supplemented with Cdh1. (D) Overexpression of myc-tagged Cdh1 induces JNK degradation in HeLa cells. Time-course refers to hours after transfection of cells with Cdh1. Graph shows a quantification of the JNK signal. (E) Cell-cycle-synchronized Cdh1 RNAi’d HeLa cells were analyzed by immunoblotting for expression levels of Cdh1 and JNK. Uncropped images for key results of this figure are shown in Figure S7.