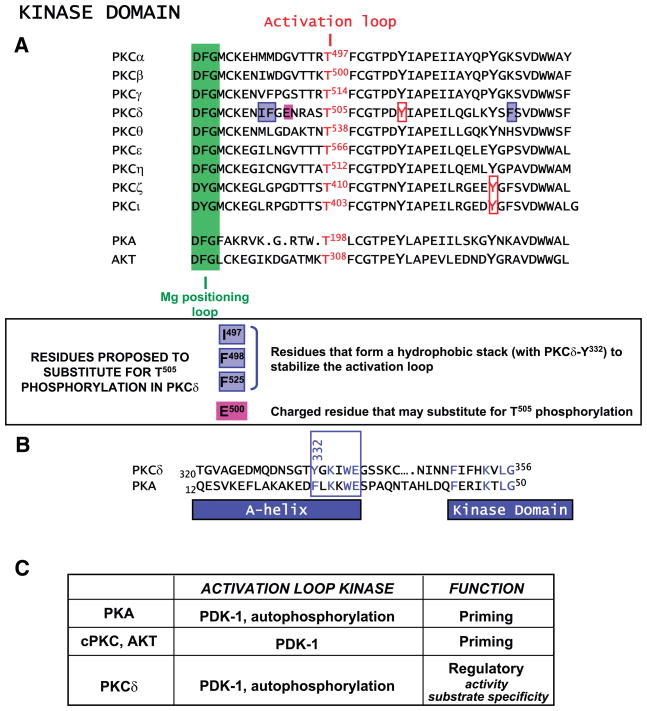
FIG. 5.
Alignment of kinase domain activation loops. A: Mg positioning loop (in green), activation loop phosphorylation site, invariant tyrosines that are phosphorylated in certain PKCs, and the unique structural features of PKCδ that recently have been considered a mechanism to render this isoform catalytically competent without activation loop phosphorylation are depicted. B: sequence alignment for the A-helix in PKCδ and protein kinase A (PKA) (see text). C: activation loop phosphorylation mechanisms and functions.