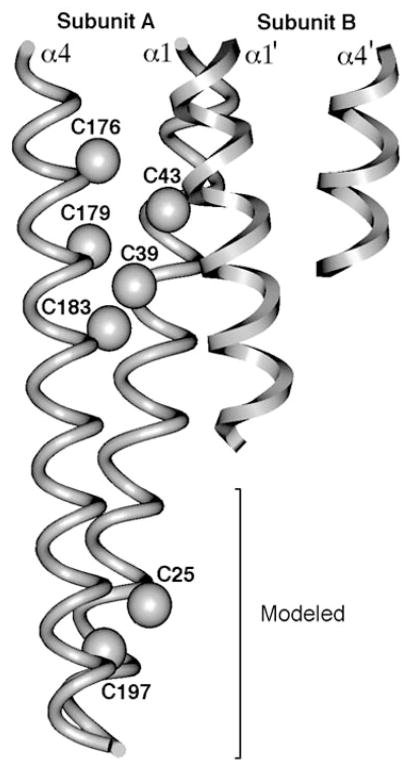
Figure 10.
Engineered cysteine pairs that yield lock-on and lock-off disulfide bonds in the full-length, aspartate receptor-kinase complex. Shown are the periplasmic ends of the four transmembrane helices in the dimer (Milburn et al 1991), two of which have been extended by modeling into the bilayer region (Chervitz & Falke 1996). A disulfide formed between cysteines Cys25/Cys197 or between Cys39/Cys183 locks the kinase on and decreases aspartate affinity. At the other extreme, a disulfide linkage between cysteines Cys176/Cys43 or between Cys179/Cys39 locks kinase activity off and increases the aspartate affinity (Chervitz & Falke 1996). These properties mirror those expected for the native on and off states of the receptor-kinase complex, respectively, in which aspartate binding causes kinase inactivation. Lock-on disulfides trap upward vertical displacements of the signaling helix; lock-off disulfides (analogous to aspartate binding) trap downward displacements toward the cytoplasm.