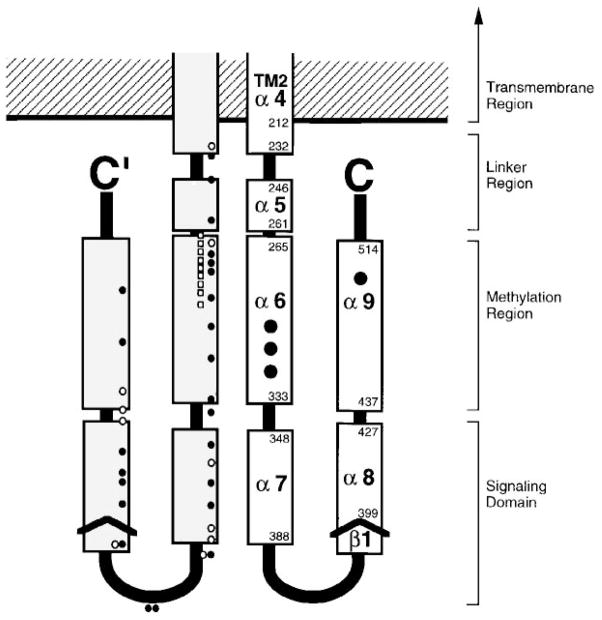
Figure 11.
Model for the cytoplasmic domain of the transmembrane receptors. A secondary-structure analysis of aligned sequences from over 56 related receptors suggests that each subunit of the homodimeric domain contains five amphiphilic helices (α5 to α9) and a short region of β-strand (β1) (LeMoual & Koshland 1996, Danielson 1997). Functionally, the domain is divided into the linker region, which provides the interface to the transmembrane signaling helix; the methylation region, which contains the sites of adaptive methylation (large black circles); and the signaling domain, which promotes CheW and CheA binding (see text for references). Also shown are the locations of lock-on and lock-off mutations in the serine receptor (white and black small circles, respectively), as well as second site suppressors of the inhibitory A19K mutation in the first transmembrane helix of the aspartate receptor (white small squares) (Ames et al 1988, Oosawa & Simon 1986). Both sets of mutations identify critical regulatory regions.