Figure 12.
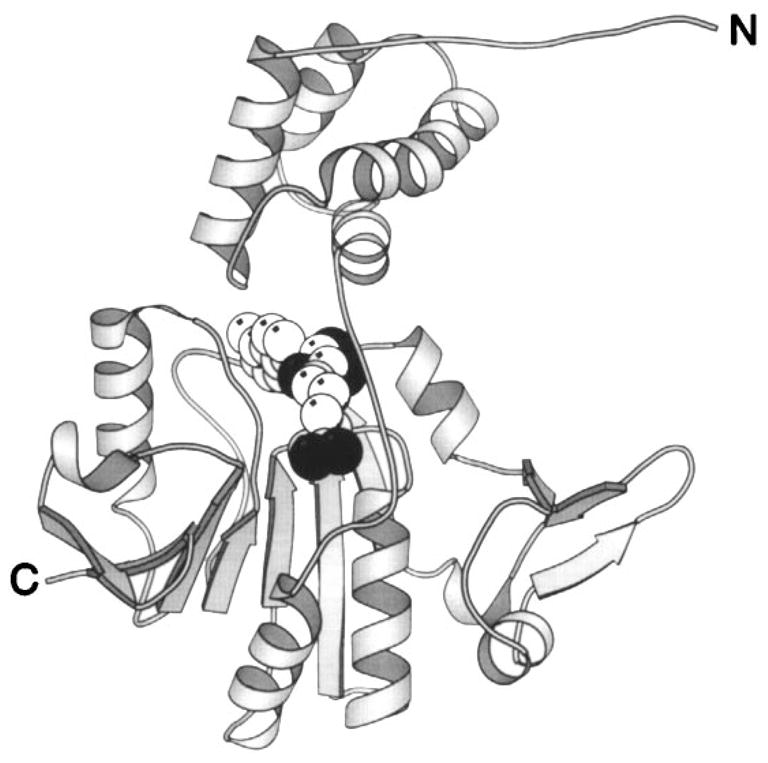
Structure of the CheR methyltransferase enzyme. The CheR protein uses S-adenosyl-methionine as a substrate for methyl transfer to the adaptation sites of transmembrane chemosensory receptors. This crystal structure reveals two distinct domains connected by a long, single-strand hinge (Djordjevic & Stock 1997). The N-terminal domain is an assembly of perpendicular helices; the C-terminal domain exhibits the α/β folding motif. The bound S-adenosyl-homocysteine molecule (CPK, sphere), a product of the methylation reaction, identifies the location of the active site region between the two domains. (Black spheres indicate oxygen atoms.)
