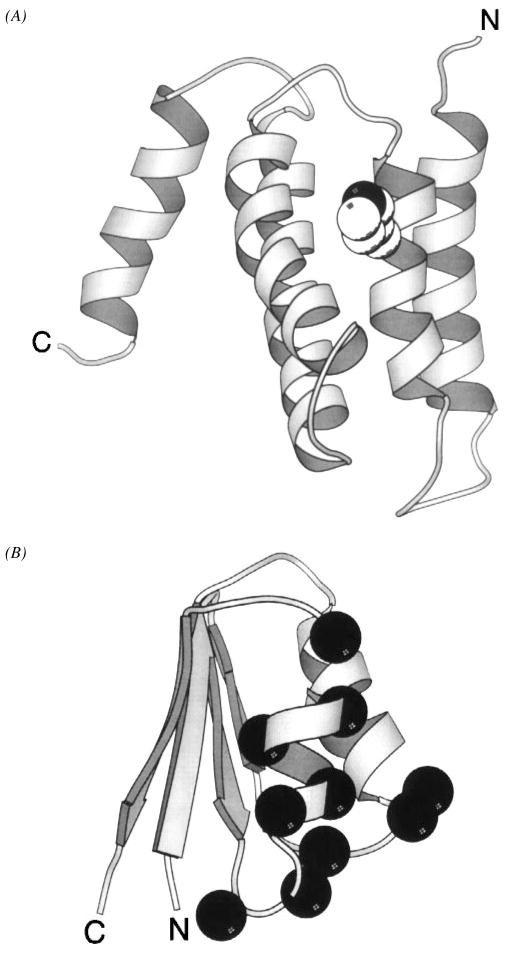
Figure 13.
Two domains of the CheA histidine kinase. (A) The N-terminal phosphotransfer domain, termed P1, provides the phospho-histidine used as a substrate during phosphotransfer from CheA to response regulators. The NMR solution structure of the phosphotransfer domain reveals a bundle of five helices (Zhou et al 1995, Zhou & Dahlquist 1997). The site of phosphorylation is the Nε2 nitrogen atom (black) of the His48 imidazole ring (CPK, sphere), located on the surface of helix α2. Phosphorylation yields a local structural change limited to the immediate environment of the phospho-histidine. (B) The solution structure of the response regulator docking domain, designated P2, displays an open-faced β-sandwich folding motif (McEvoy et al 1995, 1996). The residues implicated in CheY binding (α-carbon, black sphere) are clustered to a distinct docking surface.