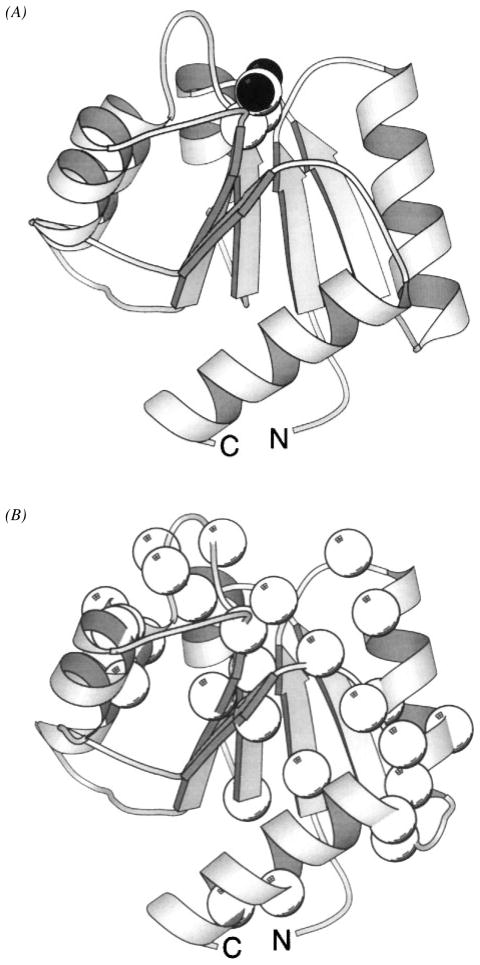
Figure 14.
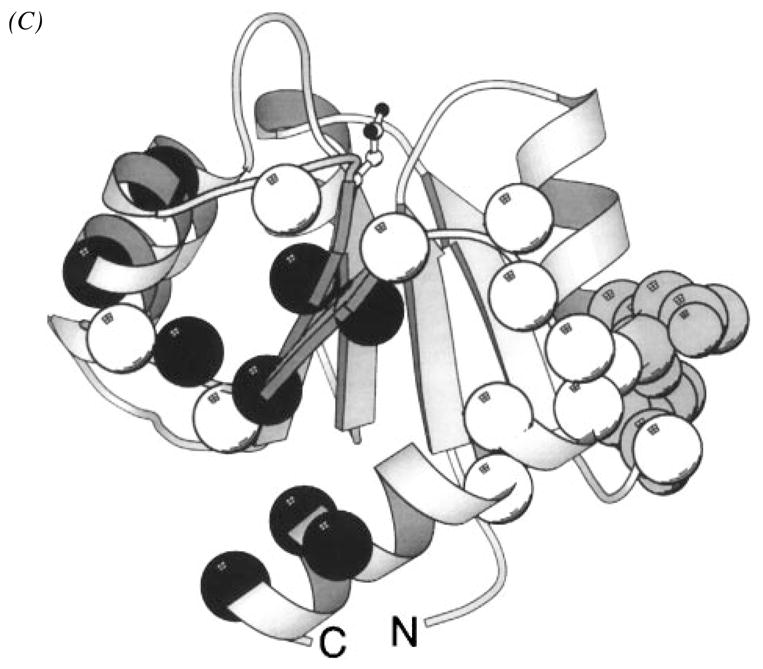
Structure of the response regulator CheY, illustrating the phospho-induced conformational regulation of three docking surfaces. (A) The CheY molecule serves as a receiver of signals from CheA and as the response regulator for motor switching. This crystal structure, which includes a bound catalytic Mg2+ ion, displays the α/β folding motif of unphosphorylated CheY (Stock et al 1993). The site of phosphorylation is Asp57 located at the upper edge of the parallel β-sheet (CPK, side chain). Other crystallographic and NMR structures have yielded the same overall backbone fold (see references in text). (B) View of CheY from the same perspective showing residues (α-carbon, sphere) perturbed by the phosphorylation-induced global conformational change, as revealed by aromatic side chain (Drake et al 1993) or backbone (Lowry et al 1994) NMR frequency changes. The large, phospho-regulated surface is seen to cover most of the protein. In addition, smaller backbone frequency changes are observed throughout the molecule, indicating that the conformational change is global (Lowry et al 1994). (C) Same perspective, illustrating a CheA docking surface defined by NMR (α-carbon, black sphere, McEvoy et al 1995, 1996) and surfaces implicated by genetic studies as important to motor switch docking (α-carbon, white sphere; Roman et al 1992, Sockett et al 1992) or CheZ interactions (α-carbon, gray sphere; Sanna et al 1995). The three regions are largely distinct, and none of the interfaces directly overlaps the phosphorylation site (Asp57 is indicated as a ball-and-stick side chain). Some overlap exists between the CheA and motor docking surfaces (Shukla & Matsumura 1995). Phospho-activation of CheY generates a global conformational change that alters the conformation of these docking regions (Drake et al 1993, Lowry et al 1994).