Figure 15.
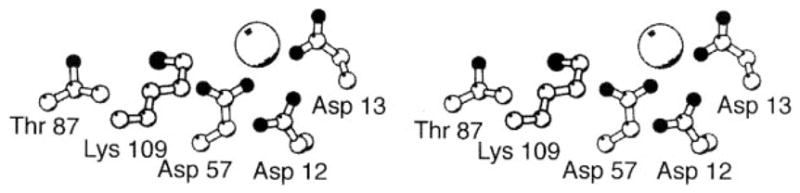
The aspartate kinase active site of CheY. Shown is the Mg2+-occupied structure of the unphosphorylated active site (Stock et al 1993), illustrating the highly conserved catalytic residues. Asp57 serves as the site of phosphorylation, and the aspartate triad (Asp12, Asp13, Asp57) provides both direct and indirect Mg2+ coordination, the latter via solvent. Lys109 and Thr87 act as acid-base catalysts. The Mg2+ ion serves as an essential cofactor in both the autocatalytic phosphorylation and dephosphorylation reactions. (For additional references, see text.)
