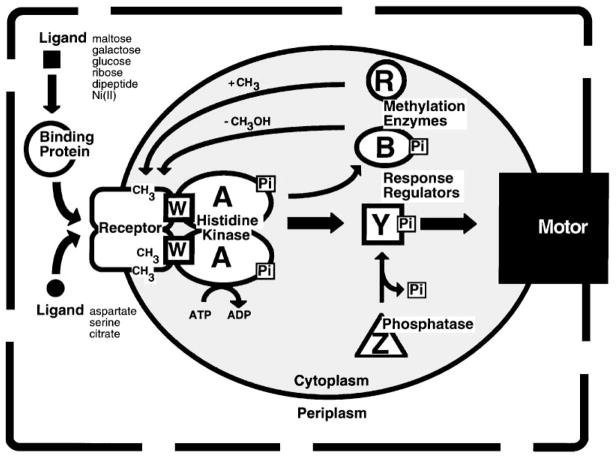
Figure 4.
The chemosensory two-component pathway of E. coli and S. typhimurium. Arrows indicate the action of one component on another. Attractants and repellents in the periplasm bind to specific transmembrane receptors or to soluble binding proteins that in turn bind to transmembrane receptors. The transmembrane receptors are coupled by a scaffolding protein (CheW) to a cytoplasmic histidine kinase (CheA), which in turn regulates two response regulators (CheB and CheY). Phosphorylation of CheB modulates the adaptation system in which CheR methylates specific regulatory glutamate side chains on the cytoplasmic surface of the receptor, whereas phospho-CheB hydrolyzes these modifications. The steady state level of receptor methylation provided by the opposing CheR and CheB reactions enables the pathway to adapt to background stimuli and also provides a simple chemical memory. Phosphorylation of CheY modulates the rotary flagellar motor as phospho-CheY docks to the motor switch apparatus, thereby controlling the direction of motor rotation and the swimming behavior of the cell. Although CheY can catalyze its own dephosphorylation, the rate of phospho-signal inactivation is enhanced by a phosphatase activity (CheZ) (see text for references).