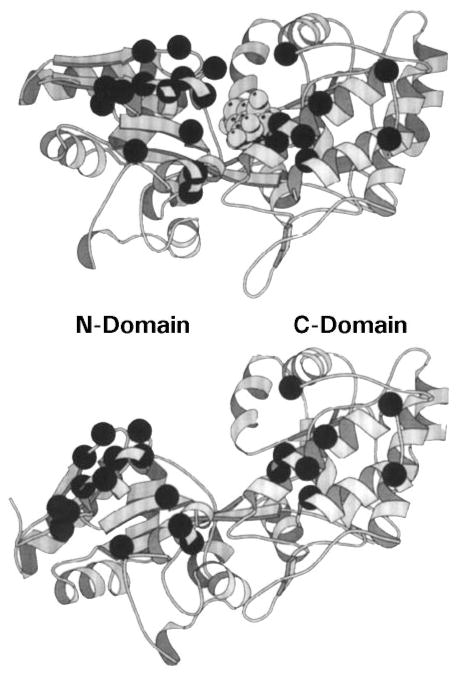
Figure 5.
Ligand-induced cleft closure in a periplasmic binding protein. Shown are crystal structures of the maltose binding protein (MBP) in its sugar-occupied (upper panel) and apo (lower panel) states (Scharff et al 1992). The ligand-binding site lies in a deep cleft separating the two domains. Bound ligand stabilizes the closed conformation of the cleft, whereas the apo cleft can open by at least 35° and also exhibits an 8° hinge twist. The structural and dynamic differences between these two states regulates the docking of binding proteins to their specific transmembrane receptors (Careaga & Falke 1992). Dark α-carbon spheres denote the genetically defined receptor-docking surface (Zhang et al 1992).