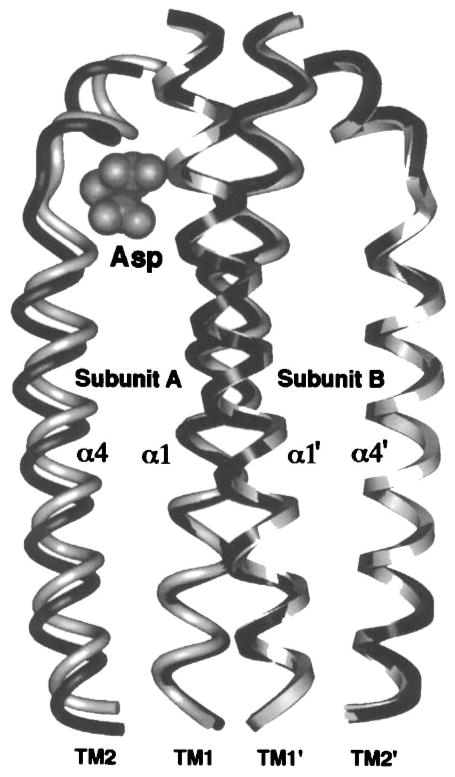
Figure 9.
The aspartate-induced displacement of the transmembrane signaling helix. Shown are the periplasmic regions of the four membrane-spanning helices, two provided by each subunit. When the apo and aspartate-occupied crystal structures (Milburn et al 1991) are superimposed using their static B subunits as a guide, aspartate is observed to displace only the α4/TM2 transmembrane helix in subunit A, termed the signaling helix (Chervitz & Falke 1996). This displacement consists of a vertical, 1.6 Å piston component directed down toward the cytoplasm as well as a subtle 5° helix tilt (difficult to visualize in this perspective). The kink or notch near the upper N-terminal end of the signaling helix is generated by conserved Pro153. This proline creates an indentation in the signaling helix complementary to the shape of the bound ligand, thereby controlling the vertical position the helix. Gray and black helices represent the apo and aspartate-occupied structures, respectively; cross-sectional shapes specify helices from subunits A (elliptical) and B (square), also denoted by primes.