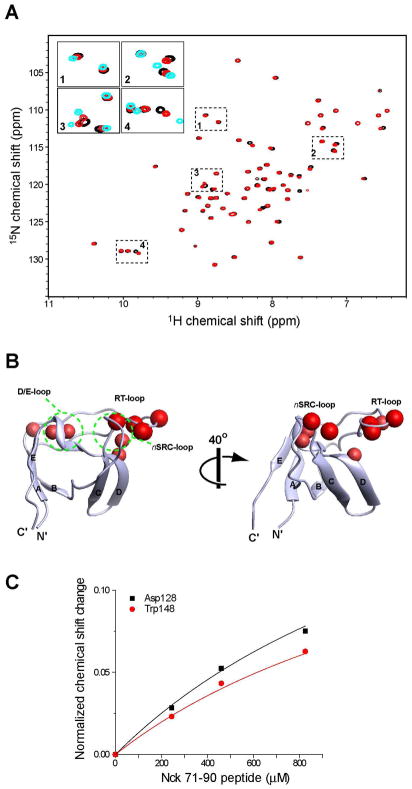
Figure 4.
Titration of Nck_71–90 peptide to the isolated SH3.2 domain. (A) Comparison of the 1H15N HSQC spectra of 100 μM [ul-15N] Nck SH3.2 with (red) or without (black) 800 μM of the Nck2_70–90 peptide. Four regions in the HSQC spectrum are enlarged as inserts. In the inserted figure, 1H15N HSQC spectrum of 100 μM [ul-15N] Nck2_70–168 is also shown in cyan. (B) Mapping of the residues that are shifted by titration of Nck_71–90. Residues that are shifted >0.2 ppm with titration 800 μM of the 71–90 peptide are indicated as red spheres, Chemical shift changes are normalized by the equation :((δN/5)2+(δH)2)1/2, where δN and δH are chemical shit changes in nitrogen and proton dimensions. The structure and orientation of the molecules are same as in Figure 3. (C) Concentration dependent chemical shift changes induced by the Nck2_71–90 peptide. Chemical shift changes for Asp128 and Trp148 with fast exchanges are shown. The maximum chemical shift change are assumed to be the difference between Nck2_70–168 and isolated SH3.2.