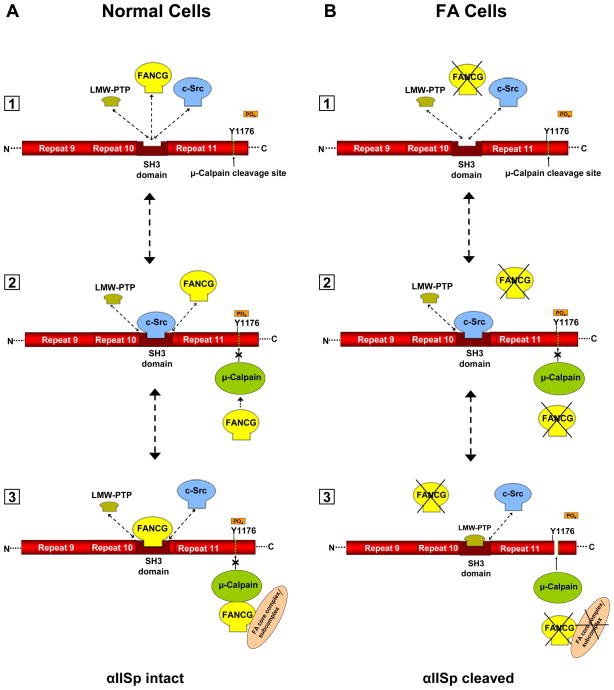
FIGURE 9.
Proposed model for involvement of FA proteins in cleavage of αIISp by μ-calpain. FANCG is used as an example in this figure; other FA proteins, such as FANCA, may be substituted into this figure. A portion of αIISp is shown containing repeats 9–11. A. [1] In normal cells an equilibrium exists between LMW-PTP, FANCG and c-Src for binding to the SH3 domain of αIISp. [2] When c-Src binds to the SH3 domain of αIISp it phosphorylates Tyr1176 (Y1176) and prevents cleavage of αIISp by μ-calpain. It also prevents binding of LMW-PTP to the SH3 domain of αIISp. [3] When FANCG binds to the SH3 domain, this prevents the binding of LMW-PTP to this site and the dephosphorylation of Tyr1176. This inhibits the ability of μ-calpain to cleave αIISp at this site, thus preventing the cleavage of αIISp. FANCG (or another FA protein) may also separately bind to calpain and inhibit its ability to cleave αIISp as shown. Both FA proteins may be FANCG, as shown, or one or the other, or both, may be FANCA or another FA protein. These proteins may also be bound to the FA core complex or to a sub-complex of the FA core proteins. B. [1] In FA cells (FA-G cells are used here as an example), there is absence of the FANCG protein and thus there is no binding of FANCG to the SH3 domain of αIISp. [2] Binding of c-Src to the SH3 domain of αIISp leads to phosphorylation Tyr1176 (Y1176) and prevents cleavage of αIISp by μ-calpain. It also prevents binding of LMW-PTP to the SH3 domain of αIISp. [3] In the absence of FANCG, LMW-PTP can bind to the SH3 domain of αIISp and dephosphorylate Tyr1176, allowing μ-calpain to cleave αIISp at its cleavage site. There is also no FANCG, or associated FA core complex or subcomplex, to bind separately to μ-calpain and inhibit its ability to cleave αIISp. This results in μ-calpain breakdown of αIISp in FA-G cells. Similar events may occur in different FA complementation groups (e.g. FA-A).