Abstract
BACKGROUND:
Despite evidence supporting the role of noninvasive ventilation (NIV) in diverse populations, few publications describe how NIV is used in clinical practice.
OBJECTIVE:
To describe NIV initiation in a teaching hospital that has a guideline, and to characterize temporal changes in NIV initiation over time.
METHODS:
A prospective, observational study of continuous positive airway pressure ventilation (CPAP) or bilevel NIV initiation from January 2000 to December 2005 was conducted. Registered respiratory therapists completed a one-page data collection form at NIV initiation.
RESULTS:
Over a six-year period, NIV was initiated in 623 unique patients (531 bilevel NIV, 92 CPAP). Compared with bilevel NIV, CPAP was initiated more often using a nasal interface, with a machine owned by the patient, and for chronic conditions, especially obstructive sleep apnea. Whereas CPAP was frequently initiated and continued on the wards, bilevel NIV was most frequently initiated and continued in the emergency department, intensive care unit and the coronary care unit. Patients initiated on bilevel NIV were more likely to be female (OR 1.8, 95% CI 1.08 to 2.85; P=0.02) and to have an acute indication compared with CPAP initiations (OR 7.5, 95% CI 1.61 to 34.41; P=0.01). Bilevel NIV was initiated more often in the emergency department than in the intensive care unit (OR 5.8, 95% CI 0.89 to 38.17; P=0.07). Bilevel NIV initiation increased from 2000 to 2005.
CONCLUSIONS:
The present study illustrates how NIV is used in clinical practice and confirms that NIV initiation has increased over time.
Keywords: Acute respiratory failure, Cohort study, Mechanical ventilation, Noninvasive ventilation, Positive pressure respiration
Abstract
HISTORIQUE :
Malgré les données probantes étayant le rôle de la ventilation non envahissante (VNI) dans diverses populations, peu de publications décrivent son mode d’utilisation en pratique clinique.
OBJECTIF :
Décrire l’amorce de la VNI dans un hôpital d’enseignement doté de lignes directrices et caractériser les changements temporels à cet égard au fil du temps.
MÉTHODOLOGIE :
Les chercheurs ont mené une étude prospective d’observation de la pression positive continue (PPC) ou de l’amorce de la VNI à deux niveaux entre janvier 2000 et décembre 2005. Des inhalothérapeutes agréés ont rempli un formulaire de collecte de données d’une page à l’amorce de la VNI.
RÉSULTATS :
Sur une période de six ans, la VNI a été amorcée chez 623 patients différents (531 VNI à deux niveaux, 92 PPC). Par rapport à la VNI à deux niveaux, la PPC était davantage amorcée à l’aide d’un dispositif nasal, d’un appareil appartenant au patient ou en réponse à une maladie chronique, notamment l’apnée obstructive du sommeil. La PPC était plus amorcée et poursuivie en milieu hospitalier, mais la VNI à deux niveaux l’était davantage à l’urgence, à l’unité de soins intensifs et à l’unité de soins coronariens. Les patients chez qui on a amorcé la VNI à deux niveaux étaient plus susceptibles d’être des femmes (RRR 1,8, 95 % IC 1,08 à 2,85; P=0,02) et d’avoir une indication aiguë que ceux chez qui on a amorcé la PPC (RRR 7,5, 95 % IC 1,61 à 34,41; P=0,01). La VNI à deux niveaux était plus fréquente à l’urgence qu’à l’unité de soins intensifs (RRR 5,8, 95 % IC 0,89 à 38,17; P=0,07). L’amorce de la VNI à deux niveaux a augmenté entre 2000 et 2005.
CONCLUSIONS :
La présente étude illustre le mode d’utilisation de la VNI en pratique clinique et confirme qu’elle est davantage amorcée au fil du temps.
Noninvasive ventilation (NIV) provides an alternative option to the initiation of invasive mechanical ventilation in patients with acute respiratory failure (ARF). It enables clinicians to provide ventilatory support to patients while instituting medical management to reverse the conditions precipitating respiratory failure. Meta-analyses strongly support the use of NIV as an initial treatment in specific etiologies of ARF including severe exacerbations of chronic obstructive pulmonary disease (COPD) (1) and congestive heart failure (CHF) (2). The evidence supporting the use of NIV – in addition to standard therapy – in patients with hypoxemic respiratory failure, is less convincing, demonstrating reductions in endotracheal intubation rate, intensive care unit (ICU) length of stay and mortality amid significant heterogeneity (3), in weaning (4) and in postextubation respiratory failure (5,6). Further evidence is required to clarify the role of NIV in these circumstances.
Despite increasing evidence supporting the role of NIV in specific populations in well-designed studies, few publications describe how NIV is actually used in clinical practice outside of the controlled clinical trial setting. Even fewer publications describe NIV use in the presence of a clinical practice guideline. Guidelines differ from protocols, which provide a set of sequential steps to standardize patient care and policies that are not necessarily based on best current evidence. Whereas, policies and protocols are often locally developed documents that involve administrative stakeholders (7), guidelines can be nationally or locally developed and modified. Guidelines represent systematically developed statements that integrate the best current evidence to guide clinicians in the care of patients for specific clinical circumstances (7).
Experiences with NIV in clinical practice may not mirror those in clinical trials because patients in clinical practice are less highly selected; interventions are applied, titrated and discontinued by staff with variable interest and expertise in their application; and monitoring is less rigorous (8). For these reasons, the results attained in clinical trials may not be realized in practice. The objectives of the present prospective, observational study were to describe how bilevel NIV is initiated in a university-affiliated teaching hospital that has a guideline for NIV initiation in place, and to characterize temporal changes in NIV initiation over time.
METHODS
Study design
A prospective, observational study of NIV initiation in a quaternary care centre over a six-year period from January 2000 to December 2005 was conducted. Episodes of NIV included initiation of continuous positive airway pressure ventilation (CPAP) or bilevel NIV. Registered respiratory therapists (RRTs) completed a one-page standardized data collection form at each NIV initiation (Appendix 1). The Research Ethics Board of the University of Western Ontario (London, Ontario) approved conduct of the present study.
Setting
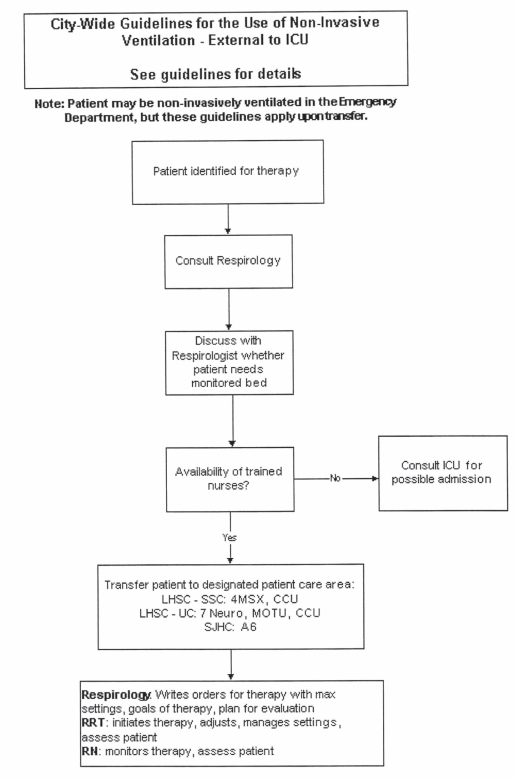
The present study was conducted in a quaternary care, university-affiliated teaching hospital in Canada. RRT members of the Department of Respiratory Therapy (London Health Sciences Centre, London, Ontario) were responsible for NIV initiation. A physician’s order was required for NIV (either CPAP or bilevel) initiation. The type of NIV initiated and the initial settings were determined either by the RRT or in collaboration with a physician. While any physician could request NIV, an institutional guideline implemented early in the study period (April 2000) mandated a consultation with either the departments of pulmonary or critical care medicine to provide direction in cases for which NIV could be initiated and continued for supportive care (Appendix 2).
Population
All patients in whom NIV (either CPAP or bilevel) was initiated were included. Patients could be included in the present study on more than one occasion if they experienced separate episodes of respiratory failure necessitating NIV during the same hospital admission or during separate hospital admissions.
Data collection
A data collection form was developed to collect demographic data and highlight features of NIV initiation including initiation location (eg, ICU, coronary care unit [CCU], extended ICU, emergency department [ED], multiorgan transplant unit, neuro-observation unit or in ‘other’ locations), and the date of initiation and discontinuation. A cardiac surgical recovery unit was opened in April 2005, and the data collection form was modified to include the cardiac surgical recovery unit as a potential site of NIV initiation. The data form documented information regarding the reason for initiation of treatment including one or more of obstructive sleep apnea (OSA), neuromuscular weakness, COPD exacerbation, chest wall deformity, CHF, central sleep apnea, central hypoventilation syndrome or ARF. Information to characterize features of NIV initiation including the initial level of inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP), or CPAP, the initial mode used (spontaneous, timed or spontaneous/timed) and the fractional concentration of oxygen used at the time of NIV initiation, were gathered. Moreover, information regarding the nature of the application (acute versus chronic), proprietary ownership of the NIV machine (patient versus hospital), the type of interface used (nasal versus face mask) and whether the admitting service or an RRT requested the consultation with either the pulmonary or critical care departments, were collected. Finally, information related to patient outcomes including intubation, death, transfer to an alternate site for continued treatment and immediate or late NIV discontinuation outcome, was recorded. One author (CH) retrospectively reviewed charts for patients with incomplete or missing data to ascertain information regarding patient outcomes.
Statistical analysis
Descriptive statistics including means (± SDs) and proportions for continuous and binary measures, respectively, were reported. The median duration of NIV use was reported with its associated interquartile range. Continuous measures were compared using the Student’s t test, and binary outcomes using either the χ2 test or Fisher’s exact test when expected values were less than five. A P≤0.05 was considered to be statistically significant. Associations between CPAP and bilevel NIV use over time were assessed using the χ2 test. First-time initiations, representing independent events in statistical analyses describing the characteristics of NIV initiation, sites of NIV initiation and continued use, and the initiation of NIV over time between groups initiated on CPAP and bilevel NIV, were considered. Finally, a multivariate analysis was performed using generalized estimating equations to compute the OR and 95% CIs of bilevel NIV initiation based on age, sex, chronicity of the clinical condition, the etiology of respiratory failure, location of initiation and year of initiation compared with CPAP initiation. All analyses were performed using SAS version 9.1 (SAS Institute, USA).
RESULTS
Over the six-year observation period, NIV was initiated on 685 occasions (588 bilevel NIV, 97 CPAP). NIV was initiated on at least one occasion in 623 patients (531 bilevel NIV, 92 CPAP initiations). Of these, NIV was initiated in 568 patients on one occasion, 49 patients on two occasions, and on three or more occasions in six patients.
Characteristics of NIV initiation
Considering all first-time NIV initiations (n=623), the average age of patients in whom bilevel NIV was initiated was significantly greater than those in whom CPAP was initiated (71.8±14.2 years versus 61.1±12.5 years, respectively). Compared with bilevel NIV, CPAP was initiated significantly more often in men than in women (50.1% versus 83.7%, respectively; P<0.001). Significant differences among the reasons for initiation of CPAP and bilevel NIV were found. While bilevel NIV was most often initiated for ARF, CHF and COPD (52.2%, 36.9% and 15.4% of first-time initiations, respectively), CPAP was largely initiated for OSA (85.9% of first-time initiations). Bilevel NIV was infrequently initiated for neuromuscular weakness, OSA, central hypoventilation, central apnea and chest wall disorders. Patients treated with bilevel NIV were initiated on a higher fractional concentration of oxygen than patients initiated on CPAP (65.8±28.8 versus 33.3±19.3; P<0.001). Average IPAP and EPAP levels used to initiate bilevel NIV were 11.5±2.3 cmH2O and 5.7±1.4 cmH2O, compared with 8.9±2.6 cmH2O in CPAP patients with significant differences in end-expiratory pressure between groups (P<0.001). Bilevel NIV was almost exclusively initiated in timed mode (99.4%). The features pertaining to NIV initiation are presented in Table 1.
TABLE 1.
Features of noninvasive ventilation initiation
| Parameter | CPAP (n=92) | Bilevel (n=531) | P |
|---|---|---|---|
| Sex, n (% male) | 77 (83.7) | 266 (50.1) | <0.001 |
| Age, years | |||
| <65 | 56 (60.9) | 136 (25.6) | <0.001 |
| 65 to 79 | 31 (33.7) | 219 (41.2) | |
| >79 | 5 (5.4) | 176 (33.2) | |
| Interface | |||
| Nasal | 76 (82.6) | 15 (2.8) | <0.001 |
| Face mask | 16 (17.4) | 516 (97.2) | |
| Machine | |||
| Patient owned | 37 (40.2) | 4 (0.7) | <0.001 |
| Hospital | 55 (59.8) | 527 (99.3) | |
| Chronic use | 78 (84.8) | 42 (7.9) | <0.001 |
| Consultation requested by | |||
| None | 3 (3.2) | 16 (3.0) | 0.25 |
| RRT | 39 (42.4) | 169 (31.8) | |
| Admitting team | 48 (52.2) | 329 (62.0) | |
| Both RRT and admitting team | 2 (2.2) | 17 (3.2) | |
Data presented as n (%) unless indicated otherwise. CPAP Continuous positive airway pressure; RRT Registered respiratory therapist
Sites of NIV initiation and continued use
Locations of NIV initiation and continued use are summarized in Table 2. Whereas CPAP was most frequently initiated and continued in ‘other’ locations, primarily on the wards, bilevel NIV was most frequently initiated and continued in the ICU, ED and CCU.
TABLE 2.
Locations of noninvasive ventilation initiation and continued use
|
Initiation |
Continued use |
|||
|---|---|---|---|---|
| Location | CPAP (n=92) | Bilevel (n=531) | CPAP (n=92) | Bilevel (n=531) |
| Emergency department | 5 (5.4) | 142 (26.7) | – | 98 (18.5) |
| ICU | 19 (20.7) | 157 (29.6) | 10 (10.9) | 207 (39.0) |
| Coronary care unit | 11 (12.0) | 94 (17.7) | 11 (12.0) | 103 (19.4) |
| Extended ICU | 1 (1.1) | 7 (1.3) | 5 (0.9) | |
| Cardiac surgery recovery unit | 2 (2.2) | 16 (3.0) | 2 (2.2) | 18 (3.4) |
| Multiorgan transplant unit | 3 (3.3) | 31 (5.8) | 2 (2.2) | 30 (5.7) |
| Neuro-observation unit | 4 (4.4) | 24 (4.5) | 3 (3.3) | 19 (3.6) |
| Other locations | 47 (51.1) | 60 (11.3) | 64 (69.6) | 51 (9.6) |
Data presented as n (%). CPAP Continuous positive airway pressure; ICU Intensive care unit
Initiation of NIV over time
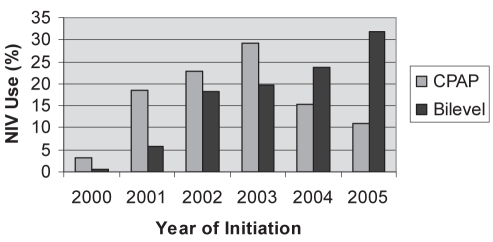
Figure 1 depicts temporal trends in CPAP and bilevel NIV initiation. While the proportion of CPAP initiations exceeded bilevel NIV initiations from 2000 to 2003, bilevel NIV was initiated more frequently than CPAP thereafter, although the rate was not constant. Regarding indication for initiation, consistent increases in bilevel NIV initiation between 2000 and 2005 were noted for episodes of ARF and exacerbations of COPD.
Figure 1).
Frequency of noninvasive ventilation (NIV) initiation over time at the London Health Sciences Centre, London, Ontario. CPAP Continuous positive airway pressure
Multivariate analysis
In the multivariate analysis, women were more likely than men to undergo bilevel NIV initiation (OR 1.8, 95% CI 1.08 to 2.85; P=0.02). Bilevel NIV was more likely to be initiated in the ED than in the ICU (OR 5.8, 95% CI 0.89 to 38.17; P=0.07) and more often initiated for acute than for chronic conditions (OR 7.5, 95% CI 1.61 to 34.41; P=0.01). Compared with CPAP initiation, significant differences were not found in bilevel NIV initiation among patients between 65 and 79 years of age, and those older than 79 years of age (using the 65 years of age and younger group as the referent category). Compared with CPAP, patients were less likely to undergo bilevel NIV initiated for OSA (OR 20, 95% CI 4.76 to 100; P≤0.001) using COPD as the referent category. Bilevel NIV was more likely to be initiated in 2005 compared with 2000 (OR 32.5, 95% CI 1.07 to 982.56; P=0.045).
Processes and outcomes
The admitting team requested either pulmonary or critical care consultations, in accordance with the institutional guideline (48 of 92 [52.2%] and 329 of 531 [62.0%] for CPAP and bilevel NIV initiation, compared with 39 of 92 [42.4%] and 169 of 531 [31.8%] for consultations requested by RRTs). A consultation was not requested by either service in 19 (6.2%) of first-time initiations. Patients were infrequently continued on bilevel NIV (110 of 531 [20.7%]) and CPAP (21 of 92 [22.8%]) during transfer to sites of continued treatment.
More bilevel-treated patients underwent early discontinuation (shortly after initiation) than CPAP-treated patients (24 of 531 [4.5%] versus two of 92 [2.2%]; P=0.30); CPAP was rarely discontinued at later time points (16 of 92 [17.4%]). Compared with CPAP-treated patients, more bilevel-treated patients required intubation (three of 92 [3.3%] versus 124 of 531 [23.4%]; P<0.001) and died (one of 92 [1.1%] versus 26 of 531 [4.9%]; P=0.10) during hospitalization.
DISCUSSION
We found several important differences in how, where and in whom CPAP and bilevel NIV were initiated in our observational study. Compared with bilevel NIV, CPAP was initiated more often using a nasal interface – with a machine owned by the patient – and for chronic conditions, especially OSA. Bilevel NIV was most often initiated for ARF, CHF and exacerbations of COPD. Whereas CPAP was most frequently initiated and continued in other locations – primarily on the wards – bilevel NIV was most frequently initiated and continued in the ICU, ED and CCU. Compared with CPAP, patients initiated on bilevel NIV were 1.8 times more likely to be female (95% CI 1.08 to 2.85; P=0.02) and 7.5 times more likely to have an acute condition (95% CI 1.61 to 34.41; P=0.01). Bilevel NIV was 5.8 times more likely to be initiated in the ED than in the ICU (95% CI 0.89 to 38.17; P=0.07) and 20 times less likely to be initiated for OSA than for CPAP (95% CI 4.76 to 100; P≤0.001). We found a significant association between bilevel NIV initiation and time, with bilevel NIV being 32.5 times more likely to be initiated in 2005 than in 2000 (95% CI 1.07 to 982.56; P=0.045) (Table 3). These findings provide insight into how bilevel NIV is initiated in a teaching hospital that has a guideline in place, and characterizes temporal changes in NIV initiation over time.
TABLE 3.
Estimates of bilevel noninvasive ventilation initiation
| Variable | OR (95% CI) | P |
|---|---|---|
| Age, years | ||
| <65 | 1.00 | – |
| 65–79 | 1.20 (0.49–2.93) | 0.686 |
| >80 | 1.57 (0.35–7.10) | 0.560 |
| Sex | ||
| Male | 1.00 | – |
| Female | 1.76 (1.08–2.85) | 0.022 |
| Condition | ||
| Chronic | 1.00 | – |
| Acute | 7.45 (1.61–34.41) | 0.010 |
| Reason for initiation | ||
| Chronic obstructive pulmonary disease | 1.00 | – |
| Obstructive sleep apnea | 0.05 (0.01–0.21) | <0.001 |
| Congestive heart failure | 2.74 (0.54–13.85) | 0.222 |
| Neuromuscular | 3.87 (0.79–18.9) | 0.094 |
| Central apnea | 0.93 (0.15–5.78) | 0.935 |
| Central hypoventilation | 1.4 (0.08–23.10) | 0.815 |
| Acute respiratory failure | 0.86 (0.25–3.00) | 0.814 |
| Initiation site | ||
| Intensive care unit | 1.00 | – |
| Emergency department | 5.82 (0.89–38.17) | 0.067 |
| Coronary care unit | 1.49 (0.44–5.00) | 0.519 |
| Extended intensive care unit stay | 0.83 (0.05–14.05) | 0.899 |
| Multiorgan transplant | 1.50 (0.46–4.87) | 0.496 |
| Neuro-observation | 1.68 (0.31–9.11) | 0.549 |
| Other | 0.40 (0.12–1.39) | 0.152 |
| Year of initiation | ||
| 2000 | 1.00 | – |
| 2001 | 8.14 (0.21–317.58) | 0.262 |
| 2002 | 17.25 (0.48–620.42) | 0.119 |
| 2003 | 16.54 (0.54–508.30) | 0.108 |
| 2004 | 9.58 (0.3–304.46) | 0.200 |
| 2005 | 32.47 (1.07–982.56) | 0.045 |
The present study has several strengths. First, it is the largest observational study conducted to date focusing on NIV initiation in practice. Second, the study highlighted important differences in how, where and in whom NIV is initiated in a teaching hospital with a guideline in place to direct its use. Third, our study demonstrated that bilevel NIV initiation increased significantly between 2000 and 2005 amid increasing literature supporting the benefits of NIV use in specific populations.
Our prospective cohort study, however, also has weaknesses. First, RRTs collected a minimum dataset on a standardized one-page data collection form each time NIV was initiated. While the minimum data set was designed to address the primary research question, it did not contain additional information to describe illness severity at the time of NIV initiation. Second, we included ARF among our indications for NIV initiation, recognizing that clinicians may be unable to accurately characterize the principal etiology of respiratory failure at the time of NIV initiation. Consequently, RRTs could have selected more than one diagnostic category at the time of NIV initiation. Our prospective data collection, therefore, mirrors the clinical uncertainty that exists in ascribing a single etiology of respiratory failure at the time of NIV initiation. Third, consistent with the research question posed, we did not limit our data collection to patients with ARF, making direct comparisons of our study population to those in the literature difficult. Finally, we retrospectively reviewed charts with missing information to ascertain information regarding outcomes. Despite attempts to ensure uniformity (eg, a single author reviewing charts), data abstraction may have been subject to recall bias.
Our results share both common features and differences with other prospective (9) and retrospective (10,11) observational studies of NIV use in clinical practice. Similar to Paus-Jenssens et al (9), who reported on 75 NIV initiations in 71 patients (bilevel NIV [85%] and CPAP [15%]), we noted that 85.8% and of all NIV initiations and 85.2% of first-time NIV initiations involved bilevel NIV. With regard to initial application, we noted that CPAP was most often initiated with a nasal mask, while bilevel NIV was usually initiated with a face mask. Our results are similar to those of Girault et al (10), who found that a face mask was the preferred interface in 81% of bilevel NIV initiations in 143 NIV episodes. Initial IPAP and EPAP levels in our study (11.5 cmH2O and 5.7 cmH2O, respectively) were also similar to those reported by Paus-Jenssens et al (9) (12 cmH2O and 6 cmH2O) and Sinuff et al (11) (10.0 cmH2O and 5.0 cm H2O).
We found that bilevel NIV was most frequently initiated in the ED and the ICU, while CPAP was most frequently initiated in other locations and the ICU. To this end, Paus-Jenssens et al (9) noted that CPAP and bilevel NIV were most often initiated in the ED (32%), a critical care setting (27%) or on general medical/surgical wards, or wards with an observation unit (41%). Whereas Sinuff et al (11) noted that bilevel NIV was predominantly initiated in the ED (62.1% of trials), we found that it was most often initiated in the ED and the ICU. Conversely, while bilevel NIV was frequently administered on clinical teaching units in their study (20.2%), we found that bilevel NIV was infrequently continued in other locations (9.5%) (11). Similar to the findings of Sinuff et al (11), in which 33.7% of patients were transferred to the ICU for bilevel NIV treatment in a postguideline implementation study, we noted that bilevel NIV was continued in the ICU in 39% of patients (12). In contrast, we observed that the majority of initiations (93.8%) had consultations with either the department of pulmonary or critical care medicine – as directed by the guideline – versus 49% of patients in their study (12).
Similar to other investigations, we found that CHF and COPD were prominent indications for bilevel NIV initiation (8,13–15). While our guideline did not specify in which patient populations NIV could be initiated, we initiated NIV in a comparatively smaller proportion of patients with diagnoses of CHF (44.2% versus 36.9%) and COPD (24.2% versus 15.4%). We postulate that this may be related to differences in data collection, with data in other studies being collected retrospectively and our data being captured at NIV initiation. Our clinical diagnoses were made in advance of continued patient observation and knowledge of response to treatment. Consequently, our indications for initiation may underestimate the prevalence of specific clinical conditions. Finally, our intubation rate of 23.4% of bilevel NIV initiations, while similar to that of Sinuff et al (11) (25.6%), is higher than that reported by Paus-Jenssens et al (9) (13%) and substantially lower than that reported by Girault et al (10) (38%). These findings suggest that NIV may be initiated in different patient populations with different illness severity at presentation. In summary, our findings are similar to those of previous investigations with regard to characteristics and frequency of NIV initiation, sites of initiation and the predominant reasons for NIV initiation, but differ in sites of continued use and outcomes achieved.
Several important observations can be made from our study. While our guideline for NIV initiation did not mandate that NIV be continued in specific locations, it prioritized consultation with either pulmonary or critical care medicine following NIV initiation. The consultative team, in collaboration with the admitting team and RRTs, assessed the patient’s clinical status, RRT accessibility and nursing expertise in deciding whether a monitored bed was required on a case-by-case basis. These decisions indicated the sites for continued NIV administration. We observed a high degree of compliance with our guideline in the requirement for a consultation, with a substantial proportion of consults requested by RRTs, and identified monitored settings for continued bilevel NIV treatment. Many guidelines exist, but fail in practice because they are not actively implemented or endorsed by end users. Our guideline provided sufficient structure to bring together key stakeholders to evaluate whether NIV could be safely continued in specific patients and locations within our hospital. In addition, it prioritized the collaborative role of admitting teams, RRTs and consultative services in working together to ensure appropriate patient disposition. Factors contributing to the successful implementation of our guideline in clinical practice include its multidisciplinary nature, and that it encouraged interaction among health care providers and was championed locally by RRTs.
Finally, our NIV initiation study confirmed that bilevel NIV initiation has increased significantly over time, although the rate has not been constant (15). Many patients in our study may have required ICU monitoring regardless of NIV initiation. Notwithstanding, while NIV use may be increasing over time, the availability of monitored beds to meet this demand has not risen commensurately. This presents clinicians involved with using NIV with a challenge that may impede appropriate and safe NIV use (16). While it may be justifiable to continue CPAP – a technology frequently managed by patients in the domiciliary setting on hospital wards – sufficient resources should be available to enable clinicians to provide bilevel NIV in areas with enhanced monitoring capacity and appropriately trained personnel. Further prospective study is required to characterize the intensity of monitoring required for patients initiated on NIV.
Acknowledgments
The authors thank the respiratory therapists at University Hospital (London, Ontario) for their dedicated efforts with data collection.
APPENDIX 1
APPENDIX 2
CCU Critical care unit; CPAP Continuous positive airway pressure; DNR Do not resuscitate; GI Gastrointestinal; ICU Intensive care unit; LHSC London Health Sciences Centre; MOTU Multiorgan transplant unit; MSX Middlesex; Neuro Neuro-observation unit; RN Registered nurse; RRT Registered respiratory therapist; SJHC St Joseph’s Health Centre; SSC South Street Campus; UC University Campus
Footnotes
CONFLICT OF INTEREST: The authors have no competing interests to declare.
DISCLOSURE: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the manuscript.
FUNDING: Dr Karen EA Burns holds a Clinician Scientist award from the Canadian Institutes of Health Research.
REFERENCES
- 1.Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbations of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med. 2003;138:861–70. doi: 10.7326/0003-4819-138-11-200306030-00007. [DOI] [PubMed] [Google Scholar]
- 2.Masip J, Roque M, Sanchez B, Fernandez R, Subirana M, Exposito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: A systematic review and meta-analysis. JAMA. 2005;294:3124–30. doi: 10.1001/jama.294.24.3124. [DOI] [PubMed] [Google Scholar]
- 3.Keenan SP, Sinuff T, Cook DJ, Hill NS. Does noninvasive positive pressure ventilation improve outcome in acute hypoxemic respiratory failure? A systematic review. Crit Care Med. 2004;32:2516–23. doi: 10.1097/01.ccm.0000148011.51681.e2. [DOI] [PubMed] [Google Scholar]
- 4.Burns KE, Adhikari NK, Meade MO. Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Review. 2003:CD004127. doi: 10.1002/14651858.CD004127. [DOI] [PubMed] [Google Scholar]
- 5.Keenan SP, Powers C, McCormick DJ, Block G. Noninvasive positive pressure ventilation for post-extubation respiratory distress: A randomized controlled trial. JAMA. 2002;287:3238–44. doi: 10.1001/jama.287.24.3238. [DOI] [PubMed] [Google Scholar]
- 6.Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452–60. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]
- 7.Sinuff T, Cook DJ. Guidelines in the intensive care unit. Clin Chest Med. 2003;24:739–49. doi: 10.1016/s0272-5231(03)00098-4. [DOI] [PubMed] [Google Scholar]
- 8.Burns KEA, Sinuff T, Adhikari NK, et al. Bilevel noninvasive positive pressure ventilation for acute respiratory failure: A survey of Ontario practice. Crit Care Med. 2005;33:1477–83. doi: 10.1097/01.ccm.0000168042.59035.d8. [DOI] [PubMed] [Google Scholar]
- 9.Paus-Jenssens ES, Reid JK, Cockroft DW, Laframboise K, Ward HA. The use of noninvasive ventilation in acute respiratory failure in a tertiary care centre. Chest. 2004;126:165–72. doi: 10.1378/chest.126.1.165. [DOI] [PubMed] [Google Scholar]
- 10.Girault C, Briel A, Hellot MF, et al. Noninvasive mechanical ventilation in clinical practice: A 2-year experience in a medical intensive care unit. Crit Care Med. 2003;31:552–9. doi: 10.1097/01.CCM.0000050288.49328.F0. [DOI] [PubMed] [Google Scholar]
- 11.Sinuff T, Cook DJ, Randall, Allen CG. Evaluation of a practice guideline for noninvasive positive-pressure ventilation for acute respiratory failure. CMAJ. 2000;163:969–73. doi: 10.1378/chest.123.6.2062. [DOI] [PubMed] [Google Scholar]
- 12.Maheshwari V, Paioli D, Rothaar R, Hill NS. Utilization of noninvasive ventilation in acute care hospitals: A regional survey. Chest. 2006;129:1226–32. doi: 10.1378/chest.129.5.1226. [DOI] [PubMed] [Google Scholar]
- 13.Sinuff T, Cook DJ, Randall J, Allen CJ. Evaluation of a practice guideline for noninvasive positive pressure ventilation for acute respiratory failure. Chest. 2003;123:2062–73. doi: 10.1378/chest.123.6.2062. [DOI] [PubMed] [Google Scholar]
- 14.Kumle B, Haisch G, Suttner SW, Piper SN, Malek W, Boldt J. Current status of NIV in German ICUs: A postal survey. Anesthesiol Intensivmed Notfall Med Schmerzther. 2003;38:32–7. doi: 10.1055/s-2003-36559. [DOI] [PubMed] [Google Scholar]
- 15.Demoule A, Girou E, Richard JS, Taille S, Brochard L. Increased use of noninvasive ventilation in French intensive care units. Intensive Care Med. 2006;32:1747–55. doi: 10.1007/s00134-006-0229-z. [DOI] [PubMed] [Google Scholar]
- 16.Vanpee D, Delaunois L, Lheureux P, et al. Survey of noninvasive positive pressure ventilation for chronic obstructive pulmonary disease in emergency departments in Belgium. Eur J Emerg Med. 2002;9:217–24. doi: 10.1097/00063110-200209000-00003. [DOI] [PubMed] [Google Scholar]