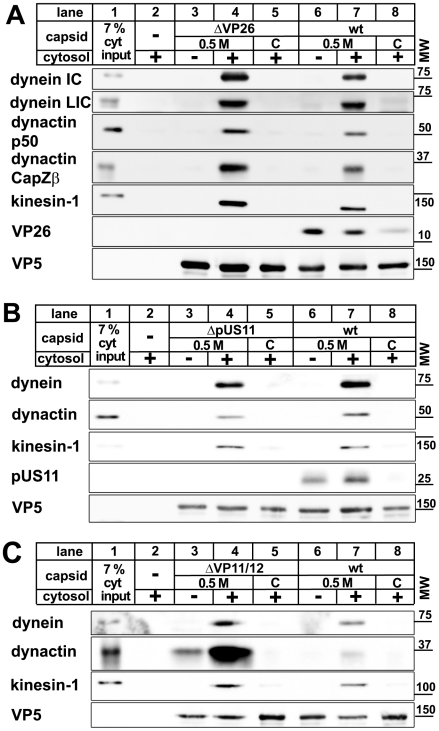
Figure 5. MAPs bind to HSV1 capsids lacking VP26, pUS11, or VP11/12.
Capsids of HSV1(KOS)-ΔVP26 (A: lanes 3 to 5), HSV1(F)-ΔUS11 (B: lanes 3 to 5), or HSV1(F)-ΔVP11/12 (C: lanes 3 to 5) were isolated by detergent lysis of trypsin-treated extracellular virions in the presence of 0.5 M KCl or from nuclei of infected cells (C capsids). HSV1(F) wild-type was used as control (A, B, C: wt, lanes 6 to 8). The capsids were incubated in 0.75 mg/ml pig brain cytosol, and after sedimentation analyzed by immunoblot with antibodies against dynein (A, B, C: MAB1618 against intermediate chain; A: pAb anti-LIC2 against light intermediate chain), dynactin (A, B: mAb anti-p50; A, C: mAb3F2.3 against CapZβ), or kinesin-1 (A, B, C: MAB1613 against heavy chain). Labeling with antibodies against VP26 (A: pAb anti-VP26) or pUS11 (B: mAb #28) confirmed the lack of these proteins on mutant capsids. As loading control, the samples were probed with antibodies against the capsid protein VP5 (A, B, C: pAB NC-1). As controls, cytosol alone was directly analyzed (A, B, C: lanes 1) or sedimented in the absence of capsids (A, B, C: lanes 2). These blots show one of two or more experiments yielding similar results.