Abstract
We investigated the contribution of frontal and parietal cortices to bottom-up and top-down visual attention using electrophysiological measures in humans. Stimuli consisted of triangles, each with a different color and orientation. Subjects were presented with a sample triangle which served as the target for that trial. An array was subsequently presented with the target and three additional distractor stimuli, which were constructed to induce either automatic “pop-out” (50%) or effortful “search” (50%) behavior. For pop-out, both the color and orientation of the distractors differed from the target, which attracted attention automatically. For search, only the orientation of the distractors differed from the target, so effortful attention was required. Pop-out target detection generated a P300 event-related potential (ERP) with a peak amplitude over parietal sites whereas the search condition generated a fronto-centrally distributed P300. Reaction times and associated P300 latency in frontal areas were shorter for pop-out targets than for search targets. We used time-frequency analysis to compare pop-out and search conditions, within a 200–650 msec time-window and a 4–55 Hz frequency band. There was a double dissociation, with significantly increased power from 4–24 Hz in parietal areas for pop-out targets and increased power from 4–24 Hz in frontal regions for search targets. Taken together the ERP and time-frequency results provide evidence that the control of bottom-up and top-down attention depend on differential contributions from parietal and frontal cortices.
Keywords: ERP, time-frequency, P300, attention, visual search, pop-out
Introduction
In daily life, humans fluidly process scenes filled with abundant information to guide behavioral selection. Attention allows one to automatically or intentionally select which aspects of the environment need to be processed and also limits the influence of distracting information (Pashler, 1998). Visual attention is controlled by both top-down cognitive factors and bottom-up sensory factors (Corbetta & Shulman, 2002). Top-down control regulates the relative signal strength of different information channels based on immediate goals and past experience. Bottom-up control acts automatically to enhance responses to biologically salient stimuli (Knudsen, 2007).
Evidence for the distinction between these two types of attention comes initially from behavioral studies in human perception (Triesman & Gelade, 1980; Egeth & Yantis, 1997). These studies indicate that, while bottom-up attention seems reflexive and automatic, top-down attention appears effortful, slow, and dependent on context (e.g. the number of distractors). In recent years, further evidence for distinguishing these processes has come from Theeuwes (1992, 2004), who has proposed that bottom-up attention always acts independently of top-down attention to automatically orient individuals toward salient but irrelevant distractors. Folk and colleagues (1992) have, however, proposed that the effectiveness of bottom-up attention can be modulated by top-down attention. Together these results suggest that while these systems may be called upon independently, they can interact to flexibly carry out two distinct attentional strategies, singleton detection and feature search, which are both relevant to survival (Bacon & Egeth, 1994). The action of these systems may occur at different times, with bottom-up attention activating early and decaying quickly and top-down attention activating at a delay and lasting longer (Theeuwes et al., 2000; Connor et al., 2004).
The control of bottom-up and top-down visual attention in humans has been investigated in a variety of functional imaging, neurophysiological and neurpsychological studies. Event-related potentials (ERPs) studies in humans have found that regions of occipital, frontal, and occipito-parietal cortex show significant changes in activity when comparing attend and ignore conditions in top-down attentional control paradigms (e.g. Clark and Hillyard, 1996). In addition, event-related functional magnetic resonance imaging (ER-fMRI) studies suggest that the intraparietal sulcus, frontal eye field, superior frontal, inferior parietal and superior temporal cortex are part of a network (frontal-parietal network) for top-down attentional control (Corbetta et al., 2000; Hopfinger et al., 2000; Hopfinger et al., 2001; Giesbrecht et al., 2003). Bottom-up attentional control seems to particularly activate regions largely lateralized to the right hemisphere and involves temporo-parietal and ventral frontal cortices (Corbetta & Shulman, 2002). Taken together, these studies provide compelling evidence of frontal and parietal cortex input into attention control (Mesulam, 1998; Kastner & Ungerleider, 2000; Sarter et al., 2001; Bledowski et al., 2004a,b; Husain & Nachev, 2007).
The contribution of frontal and parietal cortices to the control of bottom-up and top-down visual attention are often examined in separate experiments. A recent study in non-human primates assessed both types of attentional control with multiple implanted electrodes in frontal and parietal cortices (Buschman & Miller, 2007). In their match-to-sample paradigm, monkeys were required to find and focus on a visual target appearing among three distractors in separate “pop-out” and “search” conditions. As in our experiment, in the pop-out condition the color and orientation of the distractors differed from that of the target drawing attention automatically and detection time was independent of the numbers of distractors. For the search condition only the orientation of the distractors differed from that of the target, requiring a more effortful serial search (see Treisman & Gelade, 1980 for a discussion on the distinctions between serial versus parallel processing). Buschman and Miller found that during the pop-out condition neurons in the lateral intraparietal area (LIP) responded to the target first, followed by the lateral prefrontal cortex (LPFC) and the frontal eye fields (FEF). In contrast, during the search condition, neurons in the FEF and LPFC responded to the target first, followed by neurons in the LIP. Specially, frontal areas were more coherent with extrastriate areas at intermediate frequencies (22–34 Hz) during the top-down condition and parietal-extrastriate cortices were more coherent at high frequencies (35–55 Hz) during the bottom-up condition. This suggests that frontal areas are more involved in driving top-down attention, whereas neurons in more parietal areas are more involved in driving bottom-up attention. Further, these results imply that these two attentional states differ in their fundamental operations, as indicated by the different carrier frequencies.
The purpose of the current study was to compare the contribution of frontal and parietal cortices to the control of bottom-up and top-down visual attention using a similar paradigm in a human electrophysiological study. In our experiment, subjects were presented with the target stimulus and, after a delay, were shown an array consisting of the target and three distractors. Participants pressed a button when they identified the location of the target (1 for left, 2 for right) in both pop-out and search conditions (defined as in the Buschman & Miller, 2007, task above). Throughout the task, participants were instructed to maintain fixation.
In human scalp recordings, the voluntary detection of a task-relevant stimulus generates a P300 (P3b) potential 300–600 msec after a target (Picton, 1992; Polich, 2003; Polich and Criado, 2006). P300 amplitude and latency are measures of processing capacity and speed and have been linked to a variety of attentional and memory processes (see review by Kok, 1997, 2001). P300 scalp distributions are typically parietally maximal, but intracranial recordings (Halgren et al., 1995a,b; Baudena et al., 1995) and functional imaging studies (McCarthy et al., 1997; Linden et al., 1999; Clark et al., 2000) demonstrate that multiple neural sources, including prefrontal cortex, temporal-parietal junction, lateral parietal cortex, and anterior cingulate are active during P300 generation (Knight, 1997). Frontal shifts in P300 amplitude are seen in tasks that require response inhibition (the NoGo P300; Hillyard et al., 1976; Jodo & Inoue, 1990; Verleger & Berg, 1991), to distractors in harder tasks (Demiralp et al., 2001a), in serial relative to a parallel search conditions (Luck & Hillyard, 1990; 1994), and in older relative to younger subjects (Friedman et al., 1997). Taken together these results support the notion that multiple brain regions differentially engaged in a task dependent manner contribute to the scalp P300 and successful target detection, and also support the idea that the distribution of P300s are sensitive in a load-dependent manner to the activation of fronto-parietal attention control networks (Knight, 1997).
The P3b component may be influenced by different stimulus and context variables. For example, the more complex the stimulus, the larger the amplitude and the longer the latency of the P3b (Kutas et al., 1977; Johnson, 1986). However, studies using alphanumerical stimuli have reported that the amplitude of the P3b decreases with increasing memory load (Gunter et al., 1992; Kotchoubey et al., 1996; Lorist et al., 1996). Five main factors may explain this effect (Kok, 1997; Kok, 2001): larger latency jitter across trials in the high memory load, stronger equivocation in high memory load (Ruchkin and Sutton, 1978; Johnson and Donchin, 1985; Johnson, 1988), overlap between multiple P300 components or between two successive subcomponents of the P3b (Brookhuis et al., 1981; Falkenstein et al., 1994; Falkenstein et al., 1995), overlap between the P300 and slow negative waves (Okita et al., 1985; Wijers et al., 1989; Pelosi et al., 1995), and resource reallocation by the memory rehearsal component of the search task (Kramer et al., 1986; Strayer and Kramer, 1990).
ERP analyses typically measure the amplitudes and latencies of prominent event-locked peaks in the time domain. EEG/ERP frequency domain analyses have revealed that cognitive processing is functionally related to the EEG rhythms in specific frequency ranges (Demiralp et al., 2001a,b; Polich, 2007). ERSP and ERP analyses highlight similar underlying cognitive processes in different manners. For example, an auditory oddball task has been reported to generate both a large target P300 and an event-related desynchronization in the alpha frequency band (Yordanova et al, 2001). However, ERPs and ERSP analyses may also provide differentiable insights into cognitive processing (Edwards et al, 2009). Time-frequency analysis differs from ERP analysis in that it can distinguish temporally overlapping components within different frequencies and frequency overlapping components within different times. In addition, time-frequency analysis based on single trial frequency transforms is less sensitive to phase shifts or “jittering” in the EEG signal than ERPs. For these reasons, ERPs and time-frequency analysis are considered distinct measures of the signals from scalp EEG (Bastiaansen et al, 2008).
We used ERPs and time-frequency analysis to assess the role of frontal and parietal cortices in top-down versus bottom-up attention in humans. We used a paradigm based on Buschman and Miller’s (2007) study, wherein a target appeared among three distractors in separate “pop-out” and “search” conditions, and assessed the target P300 component and time-frequency spectrogram. For pop-out the distractors differed from the target in both orientation and color, such that the target drew attention automatically and for search the target differed from distractors only in orientation, requiring a more effortful search. The relative contribution of bottom-up attention is greater for the pop-out task and the relative contribution of top-down attention is greater for the search task (Treisman & Gelade, 1980), providing us with a metric to examine the neural sources of these different types of attention. We hypothesized that the pop-out and search conditions would elicit differential parietal versus frontal engagement respectively that would be visible in the distribution of the P300 component and power in the time-frequency decomposition.
Results
Behavioral Results
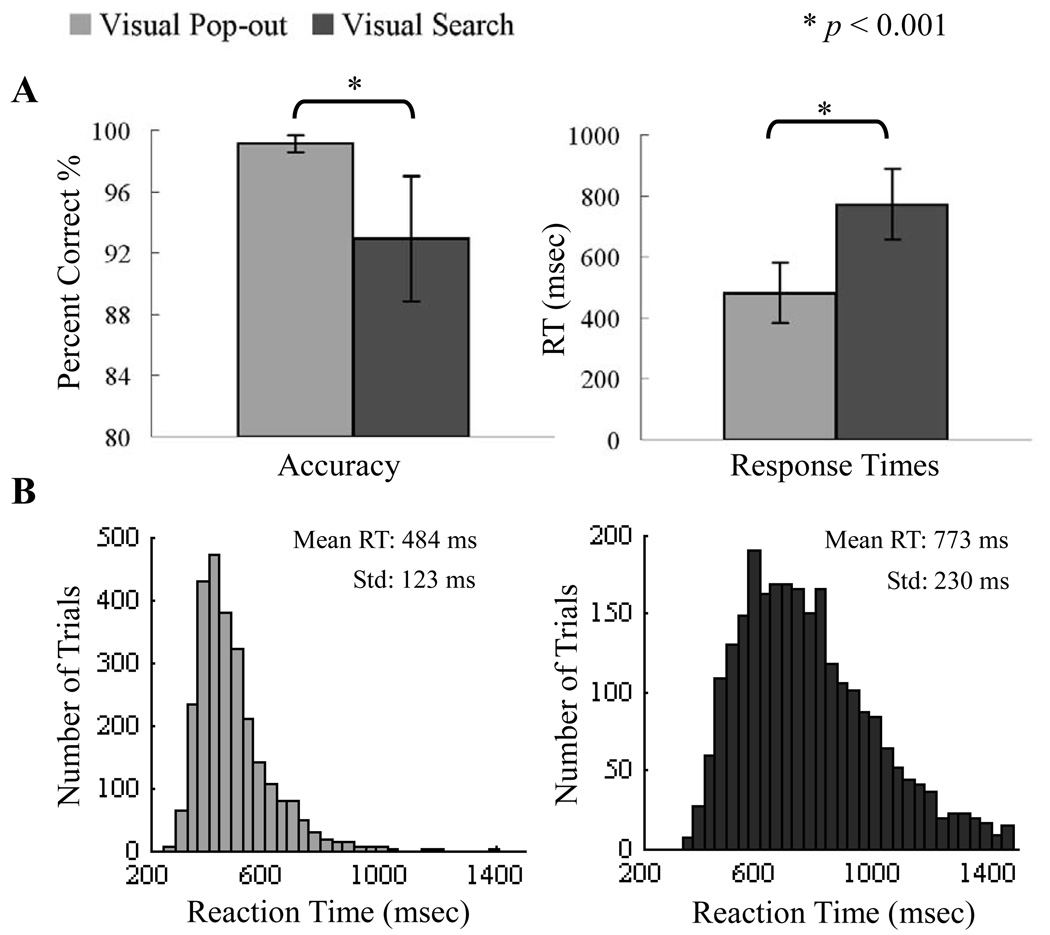
Mean accuracy was 99.12 ± 0.54% (mean ± standard deviation (Std) for this and all following results) and 92.94 ± 4.07% for the pop-out and search conditions, respectively. There was higher accuracy in the pop-out than the search condition [F(1,12) = 29.43, p < 10−4, ANOVA]. In addition, accuracy in the search condition was more variable between-subjects than accuracy in the pop-out condition [F(1,12) = 18.08, p < 10−3]. Mean reaction times (RTs) for pop-out and search conditions were 481.94 ± 99.41 msec and 772.18 ± 115.09 msec, respectively. ANOVA showed that RTs for the pop-out condition were shorter than those in the search condition [F(1,12) = 47.35, p < 10−6]. The variance in RT across all trials also differed between conditions, with a standard deviation of 123 msec for the pop-out condition and a standard deviation of 230 msec for the search condition [p < 10−5, χ2 test]. Accuracy and RT values are illustrated in Fig. 1A. All single-trial reaction times for the 13 subjects are shown in a histogram in Fig. 1B.
Fig. 1.
Behavioral Responses. (A) Average reaction time and accuracy are shown for the thirteen participants in the pop-out and search conditions. Participants responded more quickly and accurately in the pop-out condition than the search condition (* p < 0.001). (B) The histogram displays the single-trial distribution of reaction times for the pop-out (left) and search (right) conditions across all thirteen participants. Reaction times in the search condition were more variable than in the pop-out condition.
ERP Results
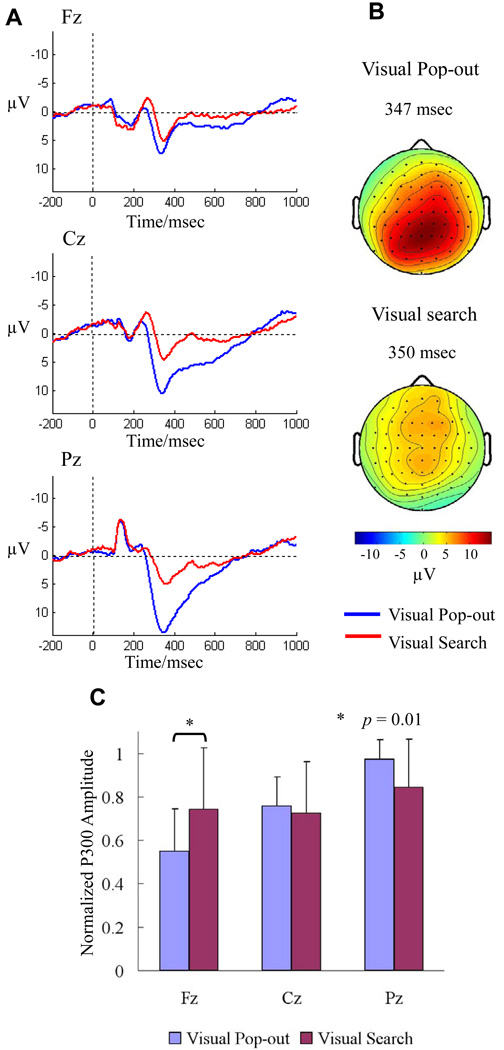
Fig. 2 presents the grand-average ERPs across all 13 subjects at Fz, Cz, and Pz electrodes (Fig. 2A), maps showing the topography of the peak P300 amplitude (Fig. 2B), and a bar graph of normalized P300 amplitude (Fig. 2C) for the pop-out and search conditions. Pop-out target detection generated a parietal maximal P300 amplitude and search target detection generated a medial fronto-central distributed P300.
Fig. 2.
(A) The P300 ERP effect is shown in a grand average across the thirteen experimental participants in three medial channels (frontal, Fz, central, Cz, and parietal, Pz), in order to show the distribution of the effect. Pop-out (blue) trials elicited significantly larger P300 components than search (red) trials across central and parietal channels, but not across the frontal channel. Note, negative is plotted up in this and all future ERP figures. (B) The distribution of the P300 effect across all 64 channels for the pop-out (top) and search (bottom) condition, at the latency of the peak amplitude of the component. The pop-out P300 was parietally distributed, whereas the search P300 was more medial central-frontally distributed. (C) The bar graph of normalized P300 amplitude for the pop-out and search conditions in Fz, Cz and Pz channels. There was a main effect of electrode (p < 0.001) and a significant interaction effect between electrode and condition (p = 0.001). Mean normalized P300 amplitude over 13 subjects for the search condition was higher than for the pop-out condition in frontal electrode (Paired t test: p = 0.01).
Repeated measures ANOVA revealed a significant difference in peak amplitude of the P3b waveform between conditions (selected as the peak between 250–450 msec) in the Cz [F(1,12) = 10.57, p = 0.003] and Pz [F(1,12) = 19.09, p = 0.001] electrodes, but no effect in the Fz [F(1,12) = 1.99, p = 0.171] electrode. Mean peak P300 amplitude over 13 subjects for the pop-out condition (Fz: 8.21 ± 3.82 µV, Cz: 11.30 ± 4.19 µV, and Pz: 14.44 ± 4.97 µV) was increasingly higher than for the search condition (Fz: 6.05 ± 3.99 µV, Cz: 5.95 ± 4.19 µV, and Pz: 6.83 ± 3.83 µV) from anterior to posterior electrodes. The P300 amplitudes over the parietal electrode were higher than over the central and frontal electrodes [Paired t tests, t(12) = 5.76, p < 10−4 between Pz and Fz; t(12) = 4.53, p < 10−3 between Cz and Fz; t(12) = 3.28, p = 0.005 between Pz and Cz] in the pop-out condition, but no such effect was present in the search condition.
The P300 amplitude was normalized individually for each condition by the peak amplitude among Fz, Cz and Pz electrodes in that condition to compare the scalp distributions of these ERP effects. A two-way ANOVA was performed on normalized P300 amplitude with electrode (Fz, Cz and Pz) and condition (pop-out and visual search) as factors to analyze the differences in the scalp distributions for pop-out and visual search conditions (Fig. 2C). There was a main effect of electrode [F(2,24) = 11.77, p < 0.001] and a significant interaction effect between electrode and condition [F(2,24) = 6.30, p = 0.001]. This result supports a difference in the scalp distributions for the two conditions. Furthermore, the mean normalized P300 amplitude for the search condition was higher than for the pop-out condition in the frontal electrode [Paired t tests, t(12) = 2.85, p = 0.01]. A second normalization procedure was used to fit the P300 amplitude to the range [−1 1]. Again, this normalized P300 amplitude for the search condition was higher than for the pop-out condition in the Fz electrode [Paired t tests, t(12) = 4.15, p = 0.0003].
There was a difference in P300 latency between the pop-out and search conditions for the Fz [F(1,12) = 7.45, p = 0.01] electrode, but no difference in the Cz [F(1,12) = 3.46, p = 0.075] and Pz [F(1,12) = 2.09, p = 0.161] electrodes. Mean P3b latency for the pop-out condition (Fz: 337.11 ± 24.18 msec, Cz: 348.83 ± 26.65 msec, Pz: 356.94 ±20.39 msec) was shorter than for the search condition (Fz: 360.47 ± 19.16 msec, Cz: 369.62 ± 30.22 msec, Pz: 369.86 ± 24.92 msec), particularly over the frontal electrode. The P300 latency over the frontal electrode was shorter than over the central and parietal electrodes [t(12) = 2.90, p = 0.01 between Fz and Cz; t(12) = 3.39, p = 0.005 between Fz and Pz] in the pop-out condition, but no such effect was present in the search condition.
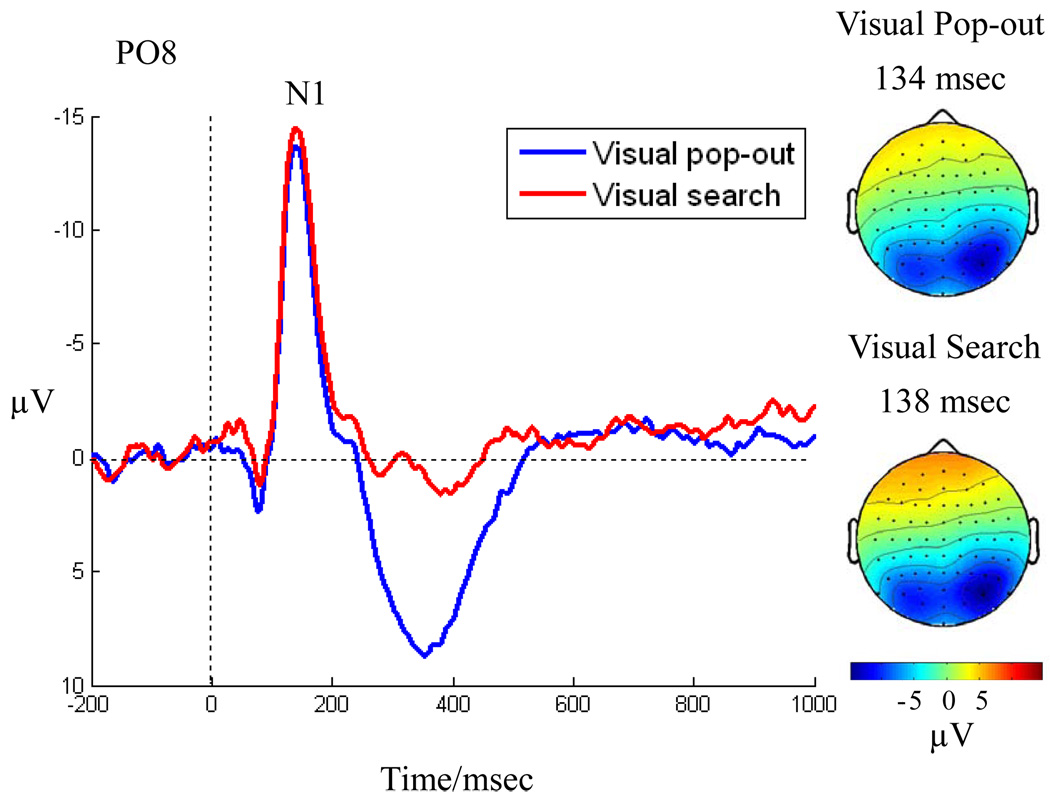
There was no significant difference at the frontal (Fz) ERP between 100–200ms. A two-way ANOVA was performed with electrode (PO7 and PO8) and condition (pop-out and search) as factors to analyze the difference in N1 peak amplitude and latency between the pop-out and search conditions. There was a main effect of electrode for the N1 peak amplitude [F(1,12) = 11.4, p = 0.001]. Mean N1 peak amplitude of the PO7 electrode (pop-out: −11.37 ± 4.48 µV, search: −11.96 ± 4.26 µV) was lower than that of the PO8 electrode (pop-out: −16.32 ± 6.44 µV, search: −16.91 ± 5.61 µV). However, there was no significant main effect of condition, or interaction between electrode and condition. N1 ERPs in the PO8 electrode are illustrated in Fig. 3 and scalp topographies of the peak amplitude over the N1 time-window are shown on the right panel. The topographical maps show a robust occipital N1 component for both conditions, with the distribution skewed toward the right hemisphere. In addition, there was a prominent P300 component in the parieto-occipital area for pop-out condition, as can be seen in Fig. 3.
Fig. 3.
The N1 ERP effect is shown as a grand average in a right posterior occipital channel (PO8). Both pop-out (blue) and search (red) elicit an N1 around 130 msec. post-stimulus onset and the two do not differ significantly. The scalp distribution of the effect is shown in the right panel across all 64 electrodes at the latency of the N1 peak. Both pop-out (top) and search (bottom) show a posterior bilateral distribution. Also visible is a prominent occipital P300 component to pop-out that is absent in the visual search condition in this channel.
Time-frequency Contribution: Distribution of Peak Power
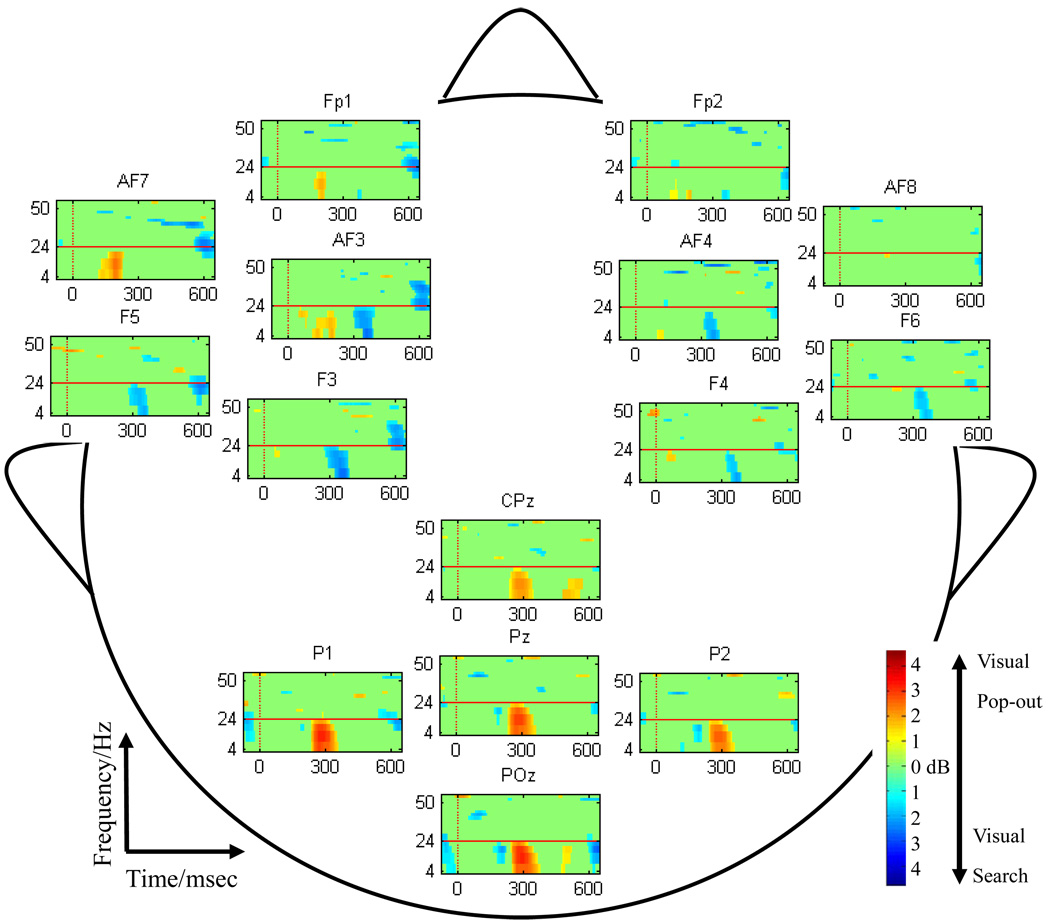
We applied event-related spectral perturbation (ERSP) analysis to compute and statistically compare transforms between the pop-out and search conditions. In the ERSP, there was significant event-related synchronization (ERS) in theta (4–8 Hz) bands within 200–400 msec and event-related desynchronization (ERD) in alpha (8–14 Hz) and beta (14–24 Hz) bands within 300–800 msec over posterior sites in both pop-out and search conditions. Fig. 4 illustrates the difference between two conditions in ERSP (pop-out – search) power in 15 electrodes (Fp1, Fp2, AF3, AF4, AF7, AF8, F3, F4, F5, F6, CPz, P1, Pz, P2, POz). Red indicates a significant increase in power for the pop-out condition compared to the search condition, and blue indicates a significant increase in power for the search condition compared to the pop-out condition. Green indicates no significant difference between the two conditions. Fig. 4 shows that the power in the P3b time-window (200–650 msec) in the pop-out condition is higher than the power of the search condition over posterior electrodes. However, the power in this same time-window is higher for the search condition than the pop-out condition over anterior electrodes. The non-parametric Chi-Square test was used to test the consistency of the ERSP results in the 13 subjects in three areas: a right frontal area (Fp2, AF4, AF8, F4, F6), a left frontal area (Fp1, AF7, AF3, F5, F3) and a parietal area (CPz, P1, Pz, P2, POz). In the parietal area, in the 200–650 msec time-window and over 4–24 Hz frequencies, the power of the pop-out condition was consistently higher than that of the search condition [p < 10−5, χ2 test], whereas there were no consistent increases in right and left frontal areas [left: p = 1, right: p = 0.5780]. Within the 200–650 msec time-window and again in the 4–24 Hz frequency band, there were consistent increases in the search condition relative to the pop-out condition in the left and right frontal areas [left: p = 0.025, right: p = 0.005], but there was no consistent increase in the parietal area [p = 0.26]. We tested the power differences between three bands: theta (4–8 Hz), alpha (8–14 Hz) and beta (14–24 Hz) in pop-out (or search) condition, but there were no significant difference among those bands in the same condition. In the 26–55 Hz frequency band, there were no consistent power differences between the pop-out and search conditions.
Fig. 4.
Example of ERSP Data from a Single Subject. The difference in power between the pop-out (more red) and search (more blue) conditions from a single subject is plotted across 15 channels, which were used for comparative distributional analyses. Over the P300 time-window (200–650 msec) pop-out elicited significantly higher power than search over medial-parietal channels, whereas search elicited significantly higher power than pop-out over lateral frontal channels. This was true over a wide band of frequencies (4–24 Hz), encompassing theta, alpha, and beta bands. There was no significant difference in the effect between frequency bands.
In order to test if the latency of the peak difference in power over parietal electrodes (for pop-out) was shorter than the latency of the peak difference in power over frontal electrodes (for search), the latencies of the peak power in the 13 subjects’ ERSP were measured within a 200–650 msec time-window and the 4–24 Hz frequency band in the parietal electrodes for the visual pop-out condition and in the frontal electrodes for the visual search condition. The mean latencies of the peak power are 296.18 ± 68.25 msec for the pop-out condition, and 431.49 ± 98.94 msec for the search condition. ANONA analysis showed that peak relative power occurred later in the search condition than pop-out condition [F(1,12) = 16.5, p = 0.0005].
Discussion
The main goal of this study was to distinguish the contributions of frontal and parietal cortices to the control of bottom-up and top-down visual attention by comparing the brain activity to pop-out and visual search target detection tasks in an EEG experiment. The behavioral data showed that participants had more rapid and accurate responses to the pop-out targets than the search targets, and the search targets evoked larger variances in RT and accuracy, indicating that the search task was sufficiently difficult to demand more cognitive effort. Both the ERP and ERSP data provide evidence that the pop-out task was associated with greater activity over parietal areas and the search task was linked to greater activity over frontal areas.
P300 Latency and Amplitude
Pop-out target detection generated a parietal maximal P300 amplitude, whereas search target detection generated a more medial fronto-centrally distributed P300, suggesting different processing by a “target detection network” in the two conditions. P300 latency in frontal areas was shorter for the pop-out condition than the search condition, in accord with the behavioral results, supporting the idea that the pop-out task was easier than the search task. Past P300 studies have shown that target P300 latency is earlier when targets are easier to find (Chao et al., 1995).
P300 amplitude in posterior regions was enhanced for the pop-out condition relative to the search condition. This fits with evidence from earlier studies showing that larger P3’s are elicited by stimuli in low versus high memory load conditions (Gomer et al., 1976; Brookhuis et al., 1981; Gunter et al., 1992; Kotchoubey et al., 1996). One previous study suggested that enlarged parietal P3 waves are elicited in “parallel” feature-present conditions relative to “serial” feature-absent conditions whereas feature-absent conditions tend to have more frontal P3s (Luck & Hillyard, 1990; 1994). Since there were no significant N1 amplitude or latency differences between the pop-out and visual search conditions, our findings suggest that the difference between the two conditions are the result of cognitive rather than perceptual load (Hillyard & Kutas, 1983).
Relationship between P300 and Time-frequency Results
We applied time-frequency analysis across single trials to further test the distinctness of the underlying neural activity in the two types of attention. There was increased power in parietal areas for pop-out targets and increased power in right and left frontal regions for search targets from 4–24 Hz. In addition, the peak relative power in the search condition occurred later than in the pop-out condition. This latter result is in accord with the shorter latency P300 in the pop-out condition relative to the search condition.
The increased P300 amplitude in parietal regions for the pop-out condition relative to the search condition was paralleled by increased power over posterior sites in the pop-out condition ERSP. This supports the hypothesis that posterior parietal cortex is primarily responsible for the automatic detection and encoding of salient stimuli (Constantinidis & Steinmetz, 2005). Furthermore, we found that the power in right and left frontal regions was higher for the search condition than for the pop-out condition, in accord with the increased normalized P300 amplitude over frontal areas in the search condition, suggesting that frontal regions are primarily involved in goal-directed target detection. Lesion, deactivation and neuroimaging studies in monkeys and humans support the contention that top-down signals related to working memory and attention are generated in prefrontal cortex (D'Esposito & Postle, 1999; Barcelo et al., 2000; Muller et al., 2002; Yago et al., 2004). The double dissociation found in the distribution of relative power in our ERSP data provides strong evidence for a dissociation in the cortical networks controlling top-down and bottom-up attention.
Delta band (0.5–4 Hz) activity within the 310–430 msec time-window has been shown to correspond to the positivity of the P300 wave using wavelet transform analysis (Demiralp et al., 1999). Increased amplitude of the delta (0.5–4 Hz) response and the theta (4–7 Hz) response are observed to targets in an oddball task (Demiralp et. al, 2001b). Event-related desynchronization (ERD) responses in the alpha (7–14 Hz) band, instead, are elicited within 400–1000 msec as measured by amplitude envelope analysis (Yordanova et al., 2001). It has been demonstrated that several partly or fully simultaneous delta, theta, and alpha components are sensitive to the same factors which elicit a P300 ERP (Yordanova et. al., 2000). In our ERSP results, frequency bands with increased power within the P300 time-window not only included theta (4–8 Hz), and alpha (8–14 Hz) bands as suggested previously, but also beta (14–24 Hz) bands over frontal and parietal sites. This suggests that the beta (14–24 Hz) band, at least under certain conditions, may also be sensitive to factors similar to those eliciting the P300.
In our experiment, low frequency bands (theta) showed ERS whereas high frequency bands (alpha and beta) showed ERD in both pop-out and search conditions. However, the changes in ERS and ERD within the P300 time-window varied by condition and electrode location. Power was relatively higher from 4–24 Hz over parietal electrodes in the pop-out condition and over frontal electrodes in the search condition. The relative increase in power across this wide frequency band could reflect either increased ERS across these frequency bands or decreased ERD or a combination of the two. Since both conditions showed ERS in low frequencies and ERD in high frequencies, the pattern of results suggests that a combination of increased ERS for low frequencies and decreased ERD for high frequencies caused the relative differences between conditions. In parietal electrodes, ERS increased more and ERD decreased less for pop-out than search conditions, whereas in frontal electrodes the opposite was true (ERS increased more and ERD decreased less for search than pop-out conditions). This suggests that P300 activity is reflective of a combined set of synchronizations and desynchronizations across frequency bands that differ in location according to the differing neural structures engaged in the task. Although Buschman and Miller (2007) found differences in the coherence between frontal and parietal cortices across different frequency bands (22–34 Hz increases for search condition and 35–55 Hz increases for pop-out condition) in their experiment, this was not evident in our EEG data, which were constrained by the skull filter and possibly the higher frequency band noise induced from muscles (Yuval-Greenberg et al., 2008).
The ERP and ERSP likely reflect components of the same underlying cognitive process. However, there is evidence to suggest that they are sensitive to distinct aspects of neural activity and may not always reflect the same underlying neural processes. In general, it has been found that ERP and ERSP results match best for low frequency bands and both tend to have a wider distribution than higher ERSP results from higher frequency bands (Edwards et al, 2009). In fact, event-related and time-frequency results can occur at different locations (Edwards et al, 2009), suggesting that they may represent distinct neural phenomena. A recent study has shown neural activity differences in the LFP due to phase, but not amplitude, changes (phase resetting with attention; Lakatos et al, 2008), providing one potential mechanism by which ERSP and ERP data could reflect different neural activity.
Role of Distinct Cortical Areas in the Control of Top-down and Bottom-up Attention
Previous studies searching for P300 generators have consistently identified the contribution of the parietal and frontal cortices to the target detection network (McCarthy et al., 1997; Linden et al., 1999; Mulert et al., 2004). In particular, the visual target P300 (P3b) is mainly produced by parietal and inferior temporal areas, whereas frontal areas and the insula regions contribute mainly to the P3a ERP (Bledowski et al., 2004a,b). This is in concordance with previous findings from lesion studies (Knight et al., 1989a, b; Yamaguchi & Knight, 1991; Verleger et al., 1994; Knight, 1997). This experiment, however, was different from the classical P300 studies typically used for generator localization, since a target was presented alongside distractor items whereas in the past studies targets and distractors were usually presented sequentially. Thus, the target P300 response in the present experiment likely has contributions from two different neural systems corresponding to two distinct modes of attention control: bottom-up and top-down. In this model, one system is associated with the saliency maps for bottom-up parietal dependent selection (Constantinidis & Steinmetz, 2005). The other system is linked to a frontal dependent goal-directed system for the detection and distinguishing of a target from distractors (Buschman & Miller, 2007).
In the current study, we found both ERP and ERSP evidence for the distinct role of the parietal and frontal cortices in top-down and bottom up attention. Together with the past literature on the sources of the P300 ERP and animal work on the networks contributing to top-down and bottom-up attention (Buschman & Miller, 2007), these results suggest that the target detection in bottom-up control has a stronger parietal contribution, and target detection in top-down control has a stronger frontal contribution and this can be measured using scalp recorded P300 and ERSP signals.
Conclusions
We used ERPs and time-frequency analysis to distinguish the effects of frontal and parietal cortices on the control of bottom-up and top-down visual attention by contrasting brain activity in pop-out and search target detection in an EEG experiment. Pop-out target detection generated a parietal maximal P300 component, whereas search target detection generated a more fronto-central distributed P300, suggesting different underlying neural sources and attention networks in the two conditions. There was increased power (4–24 Hz) in parietal areas from 200–400 msec in the pop-out condition compared to the search condition, and increased power (4–24 Hz) in frontal regions 350–650 msec for the search condition with respect to pop-out target detection. The results provide evidence that the control of bottom-up and top-down attention result from distinct processing in parietal and frontal cortices.
Experimental procedures
Subjects
Fourteen right-handed subjects (half female), age 18 to 35 (mean age = 24 years), participated in the study for monetary compensation. All the subjects had normal color vision and had no history of neurological problems. Informed written consent was obtained from all subjects prior to being tested. The Committee for the Protection of Human Subjects for the University of California, Berkeley approved the study. One subject was excluded from analyses because of excessive blinks (less than 3% trials were left); therefore, data was analyzed from the 13 remaining subjects.
Stimuli and Task
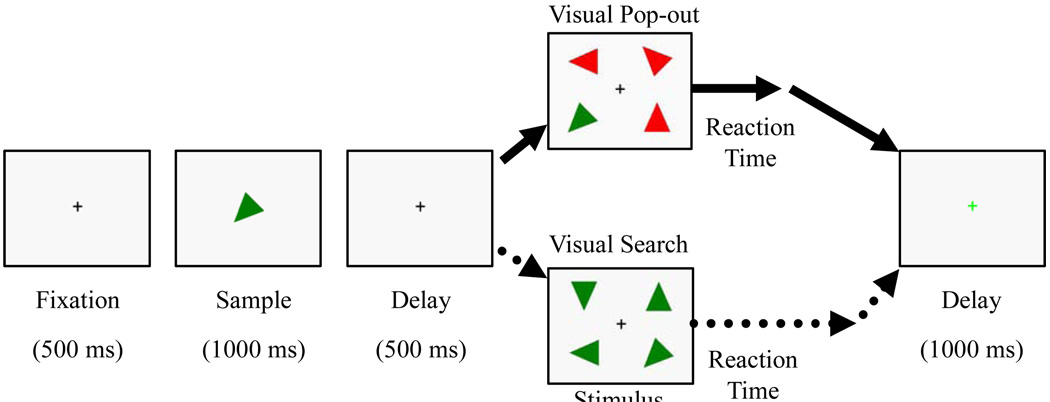
The stimuli consisted of sixteen acute isosceles triangles each with a different color and orientation combination. The length of the two equal sides of the triangle was 6.5 cm and the third side was 5.5 cm (area is 16.20 cm2). The triangles were one of two colors (red or green) and eight orientations ((i-1)×45 degree, i=1,2,3,4,5,6,7,8). Fig. 5 illustrates an example of the stimulus sequence. Trials began with a 500 msec fixation cross, after which subjects were presented with a randomly selected sample, a colored and oriented triangle, for 1000 msec, which was the target for the current trial. After the target presentation, there was a short (500 msec) delay screen consisting of the fixation cross, and then an array appeared consisting of the target and three distractors in the four quadrants of the screen. The target was randomly located in one of the quadrants. The distractors were selected to create either “pop-out” or “search” conditions. In the “pop-out” condition, the color and orientation of the three distractors differed from that of the target. In the “search” condition, however, only the orientation of the distractors differed from that of the target. The positions of the triangles were invariable, located on the upper-left, lower-left, upper-right, and lower-right, respectively. The center of each triangle was 6.2 cm. vertically, either up or down, from the center and 8.2 cm. horizontally, either right or left, from center, at a visual angle of 5.34 degrees from fixation. The array remained on the screen until the participant had responded. This was followed by a 1000 msec fixation cross, after which a new trial began.
Fig. 5.
Experimental Paradigm. Each trial began with a fixation cross (500 msec) followed by the presentation of the target (sample, 1000 msec) to remember. The target was an isosceles triangle with a particular color (red or green) and orientation (one of eight). Following the sample, there was a 500 msec delay and then the presentation of the visual array with four stimuli, composed of three distractors and the target. In the pop-out condition, the three distractors were a different color and orientation from the target, allowing for more bottom-up mechanisms of attention capture. In the search condition, instead, the distractors only differed from the target in their orientation, calling upon more controlled, top-down mechanisms of attention. The array remained on the screen until participants responded with a button press, indicating whether the target was on the left or right of the fixation cross. Following the delay, a green fixation appeared on the screen for 1000 msec, indicating the end of the trial to participants.
Subjects sat in a sound-attenuated booth with a 21-inch computer screen at a distance of 110 cm. Each trial of the task included a target; half of trials were of the search condition and half were of the pop-out condition. Subjects were required to identify which side of the screen the target appeared on. They used their right hand to press button 1 for targets appearing on the left side of the screen and button 2 for targets on the right side, regardless of whether the targets appeared on the upper or lower halves of the screen. Subjects were instructed to maintain central fixation throughout the recordings and to respond as quickly as possible without making errors. Before starting the experimental trials, subjects did two practice blocks (the first with feedback and the second without) to learn the task. Additional blocks were administered, if needed, to ensure that participants were able to achieve an accuracy of 90% for detecting the targets. Following the practice, there were twelve experimental blocks for each subject, each approximately 2.5 minutes long, consisting of 32 trials. Between blocks one minute rest was allowed, with a longer break after six blocks. In total, each subject performed 384 trials, half in the pop-out condition and half in the search condition. Stimuli were presented and behavioral results were recorded and analyzed using E-prime (Psychology Software Tools, Pittsburgh, PA).
Recording
EEG was recorded using the ActiveTwo system (Biosemi, The Netherlands) with a 64 channel electrode cap. Six additional electrodes were recorded simultaneously: the left and the right earlobes and four electrooculogram (EOG) channels. EOG were recorded from one electrode above and one electrode below the right eye to monitor vertical eye movements and one electrode lateral to each of the eyes to monitor horizontal eye movements. The EEG and EOG were amplified with an analog bandpass filter of 0.06–208 Hz and the amplified signals were digitized at 1024 Hz.
Data Preprocessing
All data processing and ERP analysis in the present paper were performed using Matlab. Raw signals were re-referenced offline to the averaged earlobes, filtered with a two-way least-squares FIR bandpass filter between 0.5 to 55 Hz (eegfilt.m from EEGLAB toolbox, Delorme & Makeig, 2004), and segmented from 200 msec before stimulus onset to 1000 msec after stimulus onset. EEG epochs containing misses (incorrect button presses or no button press within a 200 to 1500 msec window after target presentation) were excluded. EOG artifacts were rejected by a two-step procedure. First, epochs in which the difference in amplitude between the two vertical EOG signals was more than 100 µV were eliminated. Second, epochs in which the difference in amplitude between the two horizontal EOG was more than 100 µV or 3 standard deviations from of the mean EOG difference wave amplitude were rejected. For each epoch, the linear drift was removed and the data was baseline corrected using the 200 msec pre-stimulus period. If the amplitude of any channel was more than four standard deviations away from its mean over a single epoch, the epoch was excluded from further analysis. After this preprocessing, 1017 trials remained for pop-out targets and 903 trials remained for visual search targets, which were used for the further ERP and time-frequency analysis. For each subject, at least 27 trials were included in the average for each condition.
ERP Analysis
Single trials of the pop-out and search conditions were averaged with respect to the onset of the visual search array. The P300 component peak was identified as the largest positive point occurring within the 250–450 msec time-window. The peak amplitudes and latencies of the P300 were measured at the Fz, Cz and Pz electrode sites in the pop-out and search conditions. To compare the early perceptual processes between pop-out and search conditions, peak N1 amplitudes and latencies were measured during the 50–200 msec time-window at the PO7 and PO8 electrodes. The potentials at the latencies of the peak amplitude of P300 and N1 across all 64 electrodes were used to show topographical maps in each condition and to compare the scalp distributions across conditions.
The ERP amplitudes and latencies were assessed by repeated measure analysis of variance (ANOVA) between the pop-out and search conditions and paired t tests were used within condition. Chi-Square test (χ2 test) was performed to compare the variance in reaction times (RTs).
Time-frequency Analysis
The analysis of ERP components is confounded by the spatial and temporal overlap that occurs in the waveform and the variability in components that occurs across trials. For example, P300 latencies and amplitudes vary across trials and, in addition, P300 components may overlap both earlier (e.g. N2) and later (e.g. sustained positive shifts) components, making it difficult to estimate their exact size, peak, and spatial localization. The greater resistance of time-frequency analysis to phase-offsets between trials, which would normally average out of an ERP signal, was particularly important in our experiment since individuals were significantly more variable to identify the target in the search condition than the pop-out condition (see Fig. 5). Thus, a time-frequency power spectrum based on single-trial analysis was derived to extract complimentary information not revealed in the ERP. To compare the different contributions of frontal and parietal areas to the pop-out and search conditions, we used event-related spectral perturbation (ERSP) analysis to compute and statistically compare transforms between pop-out and search conditions (newtimef.m from EEGLAB toolbox, Delorme and Makeig, 2004). First, the power spectrum of a single-trial over a sliding latency window (250 msec in length with step size of 5 msec) was computed using a Fast Fourier Transform (FFT) with a Hanning window tapering. Then the common power baseline was obtained by using the mean of baseline power from both conditions for each subject. Finally, the event-related changes were computed by subtracting the baseline common power from the time-locked frequency spectrum and averaged across all trials. Significance deviations from the baseline power were evaluated using a bootstrap method (p < 0.01) (Makeig, 1993). A surrogate data distribution was produced by selecting spectral estimates for each trial from randomly selected latency windows in the specified epoch baseline, and applying this process 200 times, then averaging these data (Delorme & Makeig, 2004).
The difference in ERSP between pop-out and search conditions was calculated across all 13 subjects and 64 electrodes. Heightened power within the time-window of 200–650 msec was observed in three regions: a right frontal area (Fp2, AF4, AF8, F4, F6), a left frontal area (Fp1, AF7, AF3, F5, F3) and a parietal area (CPz, P1, Pz, P2, POz). The non-parametric Chi-Square test was used in this analysis to test the consistency across all samples.
Acknowledgements
This research was supported by grants from the NINDS NS21135, PO 40813, NSFC (No. 30800242, 30525030 and 60736029) and NSF (graduate fellowship, 2008069381). We thank Noa Fogelson for her contributions to the discussion of the P300.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bacon WF, Egeth HE. Overriding stimulus-driven attentional capture. Percept. Psychophys. 1994;55:485–496. doi: 10.3758/bf03205306. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Suwazano S, Knight RT. Prefrontal modulation of visual processing in humans. Nat. Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MCM, Mazaheri A, Jensen O. Beyond ERPs: oscillatory neuronal dynamics. In: Luck S, Kappenman E, editors. Oxford Handbook of Event-Related Potential Components. New York: Oxford University Press; 2008. [Google Scholar]
- Baudena P, Halgren E, Heit G, Clarke JM. Intracerebral potentials to rare target and distractor auditory and visual stimuli: III. Frontal cortex. Electroencephal. Clin. Neurophysiol. 1995;94:251–264. doi: 10.1016/0013-4694(95)98476-o. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Hoechstetter K, Scherg M, Wibral M, Goebel R, Linden DEJ. Localizing P300 Generators in Visual Target and Distractor Processing: A Combined Event-Related Potential and Functional Magnetic Resonance Imaging Study. J. Neurosci. 2004a;24:9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Goebel R, Zanella FE, Lindena DEJ. Attentional systems in target and distractor processing: a combined ERP and fMRI study. NeuroImage. 2004b;22:530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Brookhuis KA, Mulder G, Mulder LJM, Gloerich ABM, van Dellen HJ, van der Meere JJ, Ellerman H. Late positive components and stimulus evaluation time. Biol. Psychol. 1981;13:107–123. doi: 10.1016/0301-0511(81)90030-2. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-Down Versus Bottom-Up Control of Attention in the Prefrontal and Posterior Parietal Cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Chao LL, Nielsen-Bohlman L, Knight RT. Auditory event-related potentials dissociate early and late memory processes. Electroencephal. Clin. Neurophysiol. 1995;96:157–168. doi: 10.1016/0168-5597(94)00256-e. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R, Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. J. Neurophysiol. 2000;83:3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- Clark VP, Hillyard SA. Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. J. Cognitive Neurosci. 1996;8:387–402. doi: 10.1162/jocn.1996.8.5.387. [DOI] [PubMed] [Google Scholar]
- Connor CE, Egeth HE, Yantis S. Visual Attention: Bottom-Up Versus Top-Down. Curr. Biol. 2004;14:R850–R852. doi: 10.1016/j.cub.2004.09.041. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Posterior Parietal Cortex Automatically Encodes the Location of Salient Stimuli. J. Neurosci. 2005;25:233–238. doi: 10.1523/JNEUROSCI.3379-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat. Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:215–229. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Meth. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Schürmann M, Basar-Eroglu C, Basar E. Detection of P300 waves in single trials by the wavelet transform (WT) Brain Lang. 1999;66:108–128. doi: 10.1006/brln.1998.2027. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Comerchero M, Polich J. Wavelet Analysis of P3a and P3b. Brain Topogr. 2001a;13:251–267. doi: 10.1023/a:1011102628306. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Istefanopulos Y, Basar-Eroglu C, Basar E. Wavelet analysis of oddball P300. Int. J. Psychophysiol. 2001b;39:221–227. doi: 10.1016/s0167-8760(00)00143-4. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR. The dependence of the mnemonic components of working memory on prefrontal cortex. Neuropsychologia. 1999;37:89–101. doi: 10.1016/s0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Kim W, Dalal SS, Nagarajan SS, Berger MS, Knight RT. Comparison of time-frequency responses and the event-related potential to auditory speech stimuli in human cortex. J. Neurophysiol. 2009;102:377–386. doi: 10.1152/jn.90954.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annu. Rev. Psychol. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J. Effects of choice complexity on different subcomponents of the late positive complex of the event-related potential. Electroencephal. Clin. Neurophysiol. 1994;92:148–160. doi: 10.1016/0168-5597(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Koshlykova NA, Kiroj VN, Hoormann J, Hohnsbein J. Late ERP components in visual and auditory Go/Nogo tasks. Electroencephal. Clin. Neurophysiol. 1995;96:36–43. doi: 10.1016/0013-4694(94)00182-k. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington R, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. J. Exp. Psychol. Human. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Friedman D, Kazmerski V, Fabiani M. An overview of age-related changes in the scalp distribution of P3b. Electroencephal. Clin. Neurophysiol. 1997;104:498–513. doi: 10.1016/s0168-5597(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. NeuroImage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Gomer FE, Spicuzza RJ, O’Donnell RD. Evoked potential correlates of visual item recognition during memory scanning tasks. Physiol. Psychol. 1976;4:61–65. [Google Scholar]
- Gunter TC, Jackson JL, Mulder G. An electrophysiological study of semantic processing in young and middle aged academics. Psychophysiol. 1992;27:38–54. doi: 10.1111/j.1469-8986.1992.tb02009.x. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Liegeois C, Chauvel P, Musolino A. Intracerebral potentials to rare target and distractor auditory and visual stimuli: I. Superior temporal plane and parietal lobe. Electroencephal. Clin. Neurophysiol. 1995a;94:191–220. doi: 10.1016/0013-4694(94)00259-n. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, Vignal JP, Biraben A. Intracerebral potentials to rare target and distractor auditory and visual stimuli: II. Medial, lateral and posterior temporal lobe. Electroencephal. Clin. Neurophysiol. 1995b;94:229–250. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Courchesne E, Krausz HI, Picton TW. Scalp topography of the P3 wave in different auditory decision tasks. In: McCallum WC, Knott JR, editors. The responsive brain. Bristol UK: John Wright and Sons; 1976. pp. 81–87. [Google Scholar]
- Hillyard SA, Kutas M. Electrophysiology of cognitive processing. Ann. Rev. Psychol. 1983;34:33–61. doi: 10.1146/annurev.ps.34.020183.000341. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat. Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Woldorff MG, Fletcher EM, Mangun GR. Dissociating top-down attentional control from selective perception and action. Neuropsychologia. 2001;39:1277–1291. doi: 10.1016/s0028-3932(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Husain M, Nachev P. Space and the parietal cortex. Trends in Cogn. Sci. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Inoue K. Effects of practice on the P300 in a Go/NoGo task. Electroencephal. Clin. Neurophysiol. 1990;76:249–257. doi: 10.1016/0013-4694(90)90019-g. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr . The amplitude of the P300 component of the event-related potential: Review and synthesis. In: Ackles PK, Jennings JR, Coles MGH, editors. Advances in psychophysiology. Greenwich CT: JAI Press Inc; 1988. pp. 69–137. [Google Scholar]
- Johnson R, Jr, Donchin E. Second thoughts: Multiple P300s elicited by a single stimulus. Psychophysiology. 1985;22:182–194. doi: 10.1111/j.1469-8986.1985.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Ann. Rev. Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Knight RT. Distributed Cortical Network for Visual Attention. J. Cogn. Neurosci. 1997;9:75–91. doi: 10.1162/jocn.1997.9.1.75. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth CC. Contribution of the temporal-parietal junction to the auditory P3. Brain Res. 1989a;502:109–116. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL. Prefrontal cortex gating of auditory transmission in humans. Brain Res. 1989b;504:338–342. doi: 10.1016/0006-8993(89)91381-4. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Ann. Rev. Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Kok A. Event-related (ERP) reflections of mental resources: A review and synthesis. Biol. Psychol. 1997;45:19–56. doi: 10.1016/s0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- Kotchoubey BI, Jordan JS, Grözinger B, Westphal KP. Event-related brain potentials in a varied-set memory search task: A reconsideration. Psychophysiology. 1996;33:530–540. doi: 10.1111/j.1469-8986.1996.tb02429.x. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Schneider W, Fisk AD, Donchin E. The effects of practice and task structure on components of the event-related brain potential. Psychophysiology. 1986;23:33–47. doi: 10.1111/j.1469-8986.1986.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: The P300 as a measure of stimulus evaluation time. Science. 1977;197:792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Linden DEJ, Prvulovic D, Formisano E, Völlinger M, Zanella FE, Goebel R, Dierks T. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb. Cortex. 1999;9:815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- Lorist MM, Snel J, Kok A, Mulder G. Acute effects of caffeine on selective attention and visual search processes. Psychophysiology. 1996;33:354–361. doi: 10.1111/j.1469-8986.1996.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological evidence for parallel and serial processing during visual search. Percept. Psychophys. 1990;48:603–617. doi: 10.3758/bf03211606. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephal. Clin. Neurophysiol. 1993;86:283–293. doi: 10.1016/0013-4694(93)90110-h. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J. Neurophysiol. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jäger L, Schmitt R, Bussfeld P, Pogarell O, Möller HJ, Juckel G, Hegerl U. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. NeuroImage. 2004;22:83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Muller NG, Machado L, Knight RT. Contributions of Subregions of the Prefrontal Cortex to Working Memory: Evidence from Brain Lesions in Humans. J. Cogn. Neurosci. 2002;14:673–686. doi: 10.1162/08989290260138582. [DOI] [PubMed] [Google Scholar]
- Okita T, Wijers AA, Mulder G, Mulder LJM. Memory search and visual spatial attention: An event-related brain potential analysis. Acta Psychologica. 1985;60:263–292. doi: 10.1016/0001-6918(85)90058-7. [DOI] [PubMed] [Google Scholar]
- Pashler H. The Psychology of Attention. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Pelosi L, Hayward M, Blumhardt LD. Is “memory-scanning” time in the Sternberg paradigm reflected in the latency of event-related potentials? Electroencephal. Clin. Neurophysiol. 1995;96:44–55. doi: 10.1016/0013-4694(94)00163-f. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J. Clin. Neurophysiol. 1992;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Polich J. Overview of P3a and P3b. In: Polich J, editor. Detection of change: Event-related potential and fMRI findings. Boston: Kluwer Academic Press; 2003. pp. 83–98. [Google Scholar]
- Polich J, Criado JR. Neuropsychological and neuropharmacology of P3a and P3b. Int. J. Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118 doi: 10.1016/j.clinph.2007.04.019. 2128-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchkin DS, Sutton DS. Equivocation and P300 amplitude. In: Otto D, editor. Multidisciplinary perspectives in event-related potential research. Washington DC: US Government Printing Office; 1978. pp. 175–177. [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res. Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Strayer DL, Kramer AF. Attentional requirements of automatic and controlled processing. J. Exp. Psychol. Learn. 1990;16:67–82. [Google Scholar]
- Theeuwes J. Perceptual selectivity for color and form. Percept. Psychophys. 1992;51:599–606. doi: 10.3758/bf03211656. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Top-down search strategies cannot override attentional capture. Psychonomic Bulletin Review. 2004;11:65–70. doi: 10.3758/bf03206462. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Atchley P, Kramer AF. On the time course of top-down and bottom-up control of visual attention. In: Monsell S, Driver J, editors. Attention & performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 105–124. [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cognitive Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Verleger R, Berg P. The waltzing oddball. Psychophysiology. 1991;28:468–477. doi: 10.1111/j.1469-8986.1991.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Verleger R, Heide W, Butt C, Kompf D. Reduction of P3b potentials in patients with temporo-parietal lesions. Cognitive Brain Res. 1994;2:103–116. doi: 10.1016/0926-6410(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Wijers AA, Mulder G, Okita T, Mulder LJM. An ERP study on memory search and selective attention to letter size and conjunctions of letter size and color. Psychophysiology. 1989;26:89–109. doi: 10.1111/j.1469-8986.1989.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Yago E, Duarte A, Wong T, Barcelo F, Knight RT. Temporal kinetics of prefrontal modulation of the extrastriate cortex during visual attention. Cognitive, Affective, & Behavioral Neurosci. 2004;4:609–617. doi: 10.3758/cabn.4.4.609. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Anterior and posterior association cortex contributions to the somatosensory P300. J. Neurosci. 1991;11:2039–2054. doi: 10.1523/JNEUROSCI.11-07-02039.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Polich J. P300 and alpha event-related desynchronization (ERD) Psychophysiology. 2001;38:143–152. [PubMed] [Google Scholar]
- Yordanova J, Devrim M, Kolev V, Ademoglu A, Demiralp T. Multiple time-frequency components account for the complex functional reactivity of P300. Neuroreport. 2000;11:1097–1103. doi: 10.1097/00001756-200004070-00038. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient Induced Gamma-Band Response in EEG as a Manifestation of Miniature Saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]